‘People Are Pretty Optimistic’ as Renal Denervation’s Comeback Continues
With multiple positive pilot studies completed and pivotal trials underway, things seem to be looking up for the field.

The paper, published in the June 24, 2019, issue of JACC: Cardiovascular Interventions, is the latest attempt to understand the state of the evidence and the technological innovation in this space since the SYMPLICITY HTN-3 findings were released more than 5 years ago.
“People at that point started writing off this whole concept that some sort of device therapy could be beneficial in people with hypertension,” Michael Weber, MD (SUNY Downstate College of Medicine, Brooklyn, NY), lead author of the review, told TCTMD. “However, some of us believed that there was some merit in this whole process, and so at least three—if not more—companies started doing small clinical trials far better designed, far more focused than the earlier studies to basically do a proof of concept and demonstrate once and for all that device therapies such as renal denervation could be efficacious in patients with hypertension.”
Over the past 2 years, three sham-controlled pilot studies—SPYRAL HTN-OFF MED, SPYRAL HTN-ON MED, and RADIANCE-HTN SOLO—have reported positive results in terms of 24-hour ambulatory blood pressure changes, suggesting that efforts to improve denervation technology and technique since 2014 have been successful. Of note, however, a fourth trial—REDUCE HTN: REINFORCE—was stopped for futility.
“I think people are pretty optimistic” about how research around renal denervation is progressing, Weber said, and “now seemed to be a good time to consolidate this new information all into one place and, in a sense, set the foundation for the definitive trials that are going to be submitted ultimately to the FDA to get approval for device-based therapy, and in particular, renal denervation.”
Commenting for TCTMD, Philipp Lurz, MD, PhD, who co-authored an accompanying editorial with Karl Fengler, MD (both Heart Center Leipzig at the University of Leipzig, Germany), said, “Overall, the situation is very positive now. I think there’s no doubt that the overall principle and concept works, and there is actually a very sound body of evidence now, but everyone realizes that it still needs a little bit more to convince the skeptical ones. [It’s] always more difficult when you have a very important negative trial than without. And therefore, the next steps will be to have even larger trials and then show [a benefit on] hard clinical endpoints and if that’s successful, it’s back for good.”
Lessons Learned

The review starts by going over the rationale behind renal denervation; results from initial studies of the technology; and the failure of SYMPLICITY HTN-3 and the lessons learned.
Among them, Weber said, is that the way medical therapy was handled in the trial was not ideal, so experts convened to come up with more straightforward protocols for evaluating the effects of renal denervation both in patients with untreated hypertension and in those taking antihypertensive therapy.
In addition to improvements in how medication is handled, there have been advancements in both device technology and procedural technique. To the latter point, researchers recognized the importance of performing ablations in distal and branch renal arteries and not just the main renal arteries to achieve more complete denervation.
Referring to the pilot studies designed to incorporate these lessons, Weber said “the results are obviously promising and indicate that there is a strong justification for going now into more definitive so-called pivotal trials.” Pivotal trials of Medtronic’s Symplicity Spyral catheter, which delivers radiofrequency energy, and ReCor’s Paradise catheter, which delivers ultrasound energy, are already underway and might start producing results by late 2020, Weber said.
Additional pilot studies are ongoing as well, including the TARGET BP OFF-MED trial evaluating Ablative Solutions’ Peregrine system, which performs denervation through the injection of ethanol into the vessel wall with three needles, and two more trials of denervation using ultrasound—RADIANCE-HTN TRIO and REQUIRE.
Lingering Questions
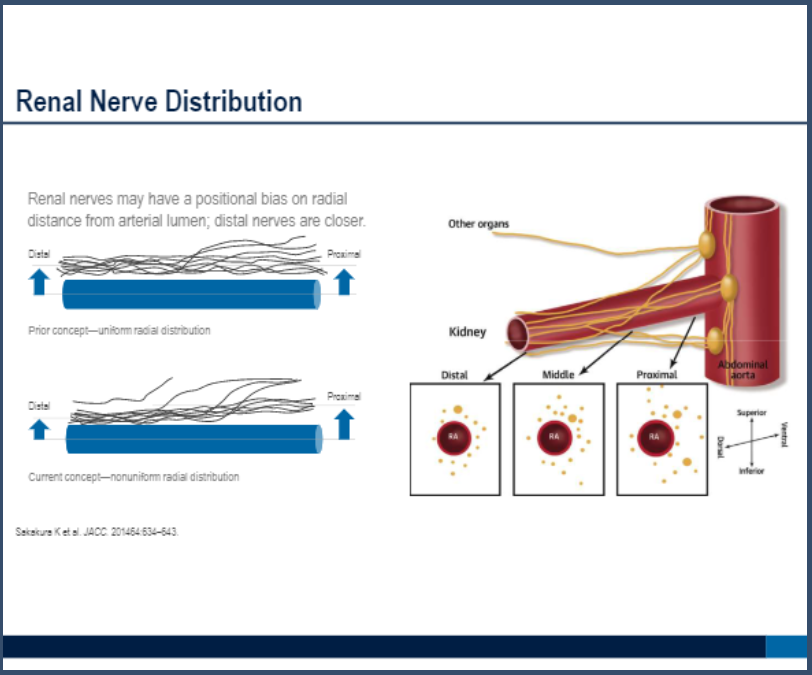
Which technology is better is unclear. A randomized pilot study called RADIOSOUND-HTN suggested that denervation by ultrasound might be better than radiofrequency ablation of the main renal arteries only, but not superior to radiofrequency ablation of the main, side, and accessory arteries.
This is a controversial topic, Weber said, adding, “I think it’s fair to say that both work and both work to a similar extent.” He noted that there is still a lot of research being done to refine the technologies.
Lurz, who a RADIOSOUND-HTN investigator, said, “I think for the next 5 or 10 years, both technologies will play a role and I don’t think that we will decide on one technology being so much better than the other and then continue with only one technology. I think they both have their strengths and [weaknesses] and maybe we’ll find out later on which patients respond to one better than to the other technology.”
I think there’s no doubt that the overall principle and concept works, and there is actually a very sound body of evidence now, but everyone realizes that it still needs a little bit more to convince the skeptical ones. Philipp Lurz
Another area of great interest to researchers is which people will be most likely to benefit from renal denervation, Weber said, who pointed out that most patients are able to keep their blood pressure under control with medication.
“It’s a relatively inexpensive, simple task to treat these patients, so why would we be considering something that’s an intervention and is clearly going to be quite a bit more expensive? And defining those patients is where the interest arises,” Weber explained. “In most cases, I suspect the procedure will be in patients who for whatever reason are not adherent, not compliant, with their drug therapy” and, thus, remain at risk for cardiovascular events.
Lurz said it will likely be an intermediate-risk cohort—those taking one to three drugs for hypertension—who will most benefit from renal denervation.
Other areas of active research, as highlighted in the review, are the search for physiological or biochemical markers that can be used to assess the completeness of denervation while the patient is still on the table and also evaluations of the durability of denervation.
Lurz agreed that an on-table measure of success and refined patient selection—with the aim of bringing the nonresponder rate down from about one-third—are important issues that need to be addressed. Moreover, he said, more research should be done to provide insights into the systemic effects of renal denervation, which are not well known; that will be key information to have when exploring the possibility of using denervation in other types of patients, such as those with heart failure or A-fib.
And future trials, Weber et al say, “will need to focus on key areas including cardiovascular outcomes following renal denervation, identification of optimal treatment populations, and cost-effectiveness.”
Weber said that renal denervation is the device therapy for hypertension that is most likely to be approved and available in the near future, adding that he thinks it will have an impact on the field of hypertension.
“I think it’ll take a couple of years after the procedure’s been approved and it’s out there for us to really come to grips with what it’s contributing in everyday clinical practice,” he said. “That’s something we still have to learn, but I would say, so far so good.”
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Weber MA, Mahfoud F, Schmieder RE, et al. Renal denervation for treating hypertension: current scientific and clinical evidence. J Am Coll Cardiol Intv. 2019;12:1095-1105.
Lurz P, Fengler K. Renal sympathetic denervation: sparks of hope with some uncertainties. J Am Coll Cardiol Intv. 2019;12:1106-1108.
Disclosures
- Weber reports having received research or consultant fees from Medtronic, Boston Scientific, ReCor Medical, Ablative Solutions, Sanofi, and Johnson & Johnson.
- Lurz reports being a consultant to ReCor Medical and Medtronic.
- Fengler reports no relevant conflicts of interest.


Comments