POLY-HF: Polypill Pays Dividends in Patients With HFrEF
The combination therapy improved LVEF, quality of life, clinical outcomes, and more, but implementation questions remain.
NEW ORLEANS, LA—A polypill containing three out of the four pillars of guideline-directed medical therapy (GDMT) improves numerous outcomes in patients who have heart failure with reduced ejection fraction (HFrEF), according to the results of the POLY-HF trial.
Compared with enhanced usual care, treatment with a capsule containing a beta-blocker, a sodium-glucose cotransporter 2 (SGLT2) inhibitor, and a mineralocorticoid receptor antagonist (MRA) led to greater improvements in LVEF, quality of life, and adherence, as well as a lower risk of a composite of HF events or death by 6 months, Ambarish Pandey, MD (UT Southwestern Medical Center, Dallas, TX), reported earlier this week at the American Heart Association (AHA) 2025 Scientific Sessions.
“These data support that simplifying medication regimens through a polypill approach can substantially improve both clinical and patient-centered outcomes in heart failure with reduced ejection fraction,” he said. “Future trials are needed to evaluate long-term effects on mortality and morbidity and assess implementation strategies to inform the adoption of polypills for management of heart failure in diverse healthcare settings.”
Orly Vardeny, PharmD (University of Minnesota, Minneapolis), the discussant for the trial, noted that “implementation efforts in recent years have largely focused on overcoming clinician inertia with strategies that promote GDMT initiation. But even if we’re able to get treatments to patients, it’s been shown that between 20 and 50% of patients are not adherent to their therapies. Thus, an intervention that dually focuses on GDMT initiation as well as medication adherence, such as the one from POLY-HF, is potentially impactful.”
In that context, the trial “adds an important piece to the implementation puzzle,” she said. But “in the end, it will take multiple parallel strategies to help ensure our patients with HFrEF get the necessary therapies that improve their quality of life and clinical outcomes.”
The POLY-HF Trial
Prior research has shown that only about 15% of patients with HFrEF who are eligible for quadruple GDMT receive it at target doses, with clinical inertia, pill burden, and other issues coming into play. One solution might be a polypill, a strategy that has proven successful for secondary prevention of atherosclerotic cardiovascular disease in the SECURE trial.
However, Pandey said at an AHA media briefing, “the challenge of using this polypill approach in heart failure is that heart failure requires complex individualized medication dosing, patients can have high risk of adverse outcomes, and it’s difficult to fit the management in a one-size-fits-all pill.”
He and his colleagues tested the concept in POLY-HF, an open-label trial conducted at two centers in Dallas. The study included 212 patients (mean age 53.5 years; 22% women) who had HFrEF (LVEF ≤ 40%) and were not on target doses of GDMT. More than half (54%) were Black, and about one-third were Hispanic. Roughly two-thirds were uninsured or had county-sponsored coverage. Mean LVEF by cardiac magnetic resonance (CMR) imaging was 26% at baseline.
Heart failure requires complex individualized medication dosing, patients can have high risk of adverse outcomes, and it’s difficult to fit the management in a one-size-fits-all pill. Ambarish Pandey
Patients randomized to the polypill arm received a gel capsule containing metoprolol succinate at one of four doses (25, 50, 100, 150 mg), empagliflozin (Jardiance; Boehringer Ingelheim/Eli Lilly) 10 mg, and spironolactone 12.5 mg, all medications that are administered once daily. Renin-angiotensin-aldosterone system (RAAS) inhibitors were administered separately to accommodate the twice-daily dosing of sacubitril/valsartan (Entresto; Novartis).
In the control arm, enhanced usual care involved the study team working with primary care physicians or cardiologists to initiate and optimize GDMT. Beta-blockers, SGLT2 inhibitors, and MRAs were provided at no cost.
Overall, the participants were relatively well treated for HF at baseline, with beta-blockers and RAAS inhibitors used in roughly 90%, MRAs in about three-quarters, and SGLT2 inhibitors in about two-thirds. Quadruple therapy at any dose was used by 44% of patients in the polypill arm and 52% of those in the control arm.
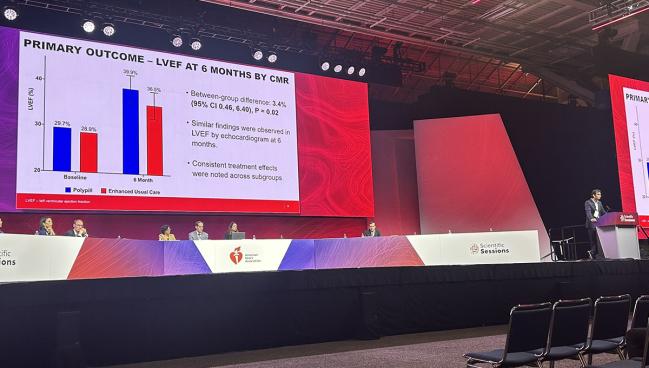
The primary outcome was the change in LVEF based on CMR imaging by 6 months. LVEF increased from 29.7% to 39.9% in the polypill arm and from 28.9% to 36.5% in the control arm, resulting in a 3.4% difference favoring the polypill (P = 0.02). The findings were similar based on echocardiographic assessments and consistent across subgroups.
The polypill strategy was associated with a lower risk of cumulative HF events (hospitalization or emergency department visits) or death at 6 months (adjusted HR 0.41; 95% CI 0.24-0.71) and an 8.5-point greater increase in the Kansas City Cardiomyopathy Questionnaire overall summary score (P = 0.005) compared with enhanced usual care. There were no significant differences between groups in NT-proBNP levels or 6-minute walk distance, although both variables numerically favored the polypill strategy.
In addition, a greater proportion of polypill-treated patients were fully adherent to treatment on the basis of therapeutic monitoring of serum drug levels (79.3% vs 54.3%; P < 0.03). By 6 months, 71% of patients in the polypill arm and 42% of those in the control arm were on quadruple GDMT at optimal doses (adjusted RR 1.68; 95% CI 1.13-2.50).
In terms of safety, in the polypill versus control arm, there were fewer cases of hyperkalemia (0 vs 4), kidney dysfunction (5 vs 11), and permanent treatment discontinuation (4 vs 18) but more cases of lightheadedness/dizziness (16 vs 6) and genitourinary infection (4 vs 0).
Implementation Challenges Remain
POLY-HF had several strengths, including the use of a pragmatic polypill formulation that preserved the physical integrity of each of the component medications, the inclusion of a racially and ethnically diverse and socioeconomically vulnerable cohort, tracking of adherence with therapeutic drug monitoring, and a comparator group in which financial obstacles to treatment were mitigated, Vardeny said.
But there are some limitations as well, she indicated, pointing to the low female enrollment, the relatively young age of the cohort compared with prior HFrEF trials, the relatively high rate of GDMT use at baseline, and the inclusion of lower-than-target doses of the beta-blocker and MRA in the polypill. Moreover, “I wonder if the polypill enabled higher usage of sacubitril/valsartan in that group, which may have favorably affected some of the outcomes,” she said.
An intervention that dually focuses on GDMT initiation as well as medication adherence, such as the one from POLY-HF, is potentially impactful. Orly Vardeny
What remains to be seen moving forward is whether a polypill for HFrEF will be accepted because it “does not address the potential baseline anxiety regarding simultaneous initiation of GDMT or hesitation with using specific components of GDMT, most notably mineralocorticoid receptor antagonists,” Vardeny said. There are also questions about whether changes in renal function, blood pressure, or electrolytes will impact long-term use of a polypill and how costs and insurance coverage will affect implementation, she added.
At the media briefing, Dorairaj Prabhakaran, MD (Centre for Chronic Disease Control, New Delhi, India), president-elect of the World Heart Federation, said survival from heart failure is worse than survival from cancer, “so bold new approaches are needed to improve evidence-based, guideline-directed medical treatment. And polypill is an attractive option.”
A host of implementation barriers will have to be overcome, and ultimately, he said, adoption of a polypill for HFrEF is going to require the buy-in of medical professionals, patients, economists, the pharmaceutical industry, governments, and international agencies like the World Health Organization, which has already added polypills to its list of essential medications.
For Mary Norine Walsh, MD (Ascension St. Vincent Heart Center, Indianapolis, IN), a past president of the American College of Cardiology, the results of POLY-HF indicate that patient engagement related to lower pill burden is a big part of the results seen. “If patients had fewer pills to take, they felt better,” she told TCTMD. “And this is a common thing that patients say, not just with heart failure but with hypertension and other things: are there any meds I can stop taking?”
A note of caution, however, involves the side effects associated with each of the components of GDMT for HFrEF, Walsh indicated, saying that dosing needs to be carefully adjusted. In that sense, she said, “I don’t think it’s a great area for a polypill.”
Although this approach may be advantageous for getting patients with newly diagnosed HF on all four pillars of GDMT at hospital discharge, Walsh said, “I’m just worried about the overall discontinuation rate because of symptoms and that you can’t really sort out what is it that’s causing the side effects.”
As for whether a polypill for HFrEF will eventually find a place in clinical practice, Walsh said “we need to study thousands of more patients” to see whether adherence lasts over the long term.
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Pandey A. Polypill for heart failure with reduced ejection fraction: the POLY-HF trial. Presented at: AHA 2025. November 10, 2025. New Orleans, LA.
Disclosures
- The trial was funded by the National Institute on Minority Health and Disparities.
- Pandey reports receiving research support from the American Heart Association, Anumana, AstraZeneca, the National Institutes of Health, Roche Diagnostics, scPharmaceuticals, SQ Innovation, and Ultromics; consulting for Northwestern University, Tricog Health, Lilly USA, Rivus, Cytokinetics, Roche Diagnostics, Sarfez Therapeutics, Edwards Lifesciences, Merck, Bayer, Anumana, Novo Nordisk, Alleviant, Pfizer, Abbott, iRhythm, Axon Therapies, Kilele Health, Acorai, Ultromics, Kardigan, Novartis, Idorsia Pharma, and Science37; and receiving stock in exchange for consulting for Palomarin.
- Vardeny reports receiving research support to her institution from AstraZeneca, Bayer, Cardurion, and CPC and personal consulting fees from AstraZeneca, Bayer, Cardior, Cytokinetics, Moderna, and Novo Nordisk.
- Prabhakaran reports no relevant conflicts of interest.





Comments