SCOUTing the ‘Forgotten Valve’: Promise for a New Percutaneous Treatment Option in Tricuspid Regurgitation
The first challenge in treating tricuspid regurgitation will be reeducating physicians to pay attention and refer patients earlier, experts say.
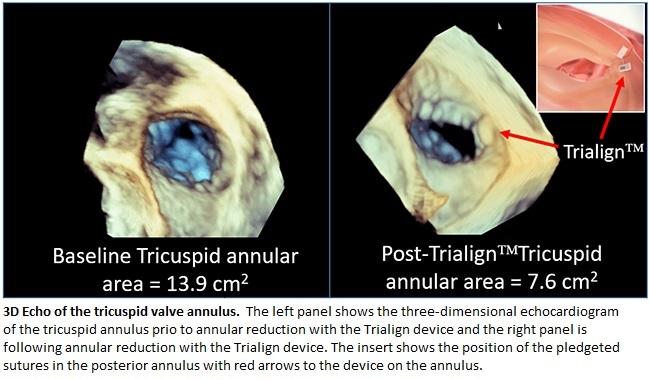
Early feasibility data on a new percutaneous option for treating severe tricuspid regurgitation confirms the safety of the procedure while suggesting it can improve quality of life for patients who have previously had no other treatment alternatives.
The Trialign system (Mitralign, Tewksbury, MA) was originally developed to treat mitral regurgitation using pledgeted sutures, but it was reconfigured to fit in the tricuspid valve using the surgical predicate of a modified Kay procedure. It is not the first device adapted to treat tricuspid disease: as recently reported by TCTMD, researchers have explored using the MitraClip (Abbott) in tricuspid valve disease and other devices are in development. Yet according to Rebecca Hahn, MD (New York-Presbyterian/Columbia University Medical Center, New York), who led the current study, researchers involved in treatments for tricuspid regurgitation are facing “an uphill climb” with regard to reeducating physicians about paying attention to this valve and seeking treatment before it’s too late.
Results for the SCOUT trial were published in the April 11, 2017, issue of the Journal of the American College of Cardiology.
Hahn told TCTMD that SCOUT was the first early feasibility study of its kind conducted entirely in the United States—a notable accomplishment, she told TCTMD, since these studies are usually completed in Europe. The researchers enrolled 15 patients with NYHA functional class ≥ II and what Hahn called “torrential tricuspid leaking” to receive treatment with the Trialign system. Four centers participated in the study.
All patients received successful device implantation with no periprocedural complications. At 30 days, technical success was 80%; three patients reported single-pledget annular detachments not requiring reintervention. However, in the remaining 12 patients compared with baseline, echocardiography showed significant reductions in tricuspid annulus area (12.3 ± 3.1 cm2 to 11.3 ± 2.7 cm2; P = 0.019) and effective regurgitant orifice area (0.51 ± 0.18 cm2 to 0.32 ± 0.18 cm2; P = 0.020), as well as an increase in LV stroke volume (63.6 ± 17.9 mL to 71.5 ± 25.7 mL; P = 0.021).
Among the entire cohort, patients showed improvement in NYHA functional class (all improved by ≥ 1 class; P = 0.001), the Minnesota Living with Heart Failure Questionnaire (mean score of 47.4 to 20.9; P < 0.001), and the 6-minute walk test (mean distance of 245.2 to 298.0 meters; P = 0.008).
Take Heed, Treating Physicians
“Tricuspid regurgitation is really understudied, and everyone refers to it as the forgotten valve because in the past you were able to take out the tricuspid valve for endocarditis for instance and just leave the patient with severe tricuspid regurgitation,” Hahn explained. “But longitudinal studies and natural history studies show pretty clearly that leaving tricuspid regurgitation of almost any severity has some impact on survival.”
Given the lack of guidelines for the treatment of tricuspid disease, the vast majority of patients are treated with medication “and then at some point the medications stop working,” she continued, adding that at this point, the tricuspid regurgitation is so severe that even a percutaneous option might not help. So the biggest challenge moving forward will be identifying patients for treatment earlier, provided that devices like Trialign and others continue to move forward and operator experience grows.
“We have to get to the medical schools and the residency programs in order to retrain the physicians to be thinking about the tricuspid valve and to refer these patients earlier,” Hahn said. “We are slowly chipping away at it. The attention . . . that the tricuspid valve has gotten in the interventional world has just exploded exponentially. That will filter down to the cardiologists and the internists, and the hope is that these subtle symptoms that are indicators of early disease will not be ignored.”
In an editorial accompanying the study, Azeem Latib, MD (San Raffaele Scientific Institute, Milan, Italy), and Antonio Mangieri, MD (EMO-GVM Centro Cuore Columbus, Milan, Italy), agree that the tricuspid valve has traditionally been “virtually ignored,” and highlight how researchers in this field can benefit from past lessons learned by operators who’ve done transcatheter mitral valve as well as surgical tricuspid valve procedures.
Specifically, Latib told TCTMD that, as Hahn suggested, changing the mindset of treating physicians must come first. “If we could get patients a little bit earlier, some of the other challenges . . . about anatomical criteria and patient selection would actually be easier, because we would be getting patients who were symptomatic with severe tricuspid regurgitation but are not end-stage tricuspid regurgitation,” he said.
Additionally, looking back to when the MitraClip was in its infancy, “we had no standardized imaging,” Latib said. “Now it’s become standardized. It's something that many physicians can do. We need the same thing with the tricuspid valve.”
Francesco Maisano, MD (UniversitätsSpital Zürich, Switzerland), who was not involved in the study, pointed to other aspects of tricuspid valve research that will help advance the field. For one, determining standardized endpoints will be important for future tricuspid valve device trials, as will an academic research consortium document, similar to what was done with TAVR and mitral valve procedures with VARC and MVARC, respectively, he said.
Hahn said the first meetings for TVARC have indeed been scheduled for the coming months, but she foresees additional challenges for defining endpoints because of the lack of research on the tricuspid valve. Still, “the need for such a document is very high,” she said.
‘A Lot of Advantages’
As for how Trialign compares with the other potential options out there, Maisano said he likes the platform. “It is a tool, a plication device, just like the clip is a leaflet fastening device. These tools can be used with creativity in different applications. However, they require some experience from the operators,” he explained in an email.
Latib said the device “has a lot of advantages” in that it reproduces the surgical technique, it is safe, and “the actual footprint, or what the implant leaves behind, is really small,” leaving open the option of retreatment if necessary.
However, Ahmed El-Eshmawi, MD (Mount Sinai Medical Center, New York, NY), also commenting on the study, offered some caveats. For one, just how improvements with quality of life and echocardiographic parameters stack up against optimal medical therapy is not clear, he said in an email. In the future, he added, “it will not be surprising” to see various transcatheter devices used in combination with each other, and this route might even be superior to the use of one device. The Trialign system might “even extend to address the [tricuspid valve] at the same time the of the established left-sided transcatheter valve therapy,” El-Eshmawi suggested.
While the future leaves much to be discovered, one thing that is clear is that the tricuspid is “no longer the forgotten valve,” El-Eshmawi concluded.
Photo Credit: Rebecca Hahn
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Hahn RT, Meduri CU, Davidson CJ, et al. Early feasibility study of a Transcatheter tricuspid valve annuloplasty: SCOUT trial 30-day results. J Am Coll Cardiol. 2017;69:1795-1806.
Latib A, Mangieri A. Transcatheter tricuspid valve repair: new valve, new opportunities, new challenges. J Am Coll Cardiol. 2017;69:1807-1810.
Disclosures
- Hahn reports serving as a speaker for Edwards Lifesciences, Abbott Vascular, Boston Scientific, and GE Medical; serving as an unpaid national principal investigator for the SCOUT trial; and serving as an uncompensated director of Echo Core for multiple industry-sponsored trials.
- Latib reports serving as a consultant to 4-Tech, Mitralign, Millipede, and Valtech Cardio and receiving speaker honoraria from Abbott Vascular.
- Mangieri, Maisano, and El-Eshmawi report no relevant conflicts of interest.


Comments