Stick-on Sensor Predicts Heart-Failure Hospitalizations: Pilot Study
The wearable patch, combined with machine learning, generated the same type of warnings sought by several implantable devices.
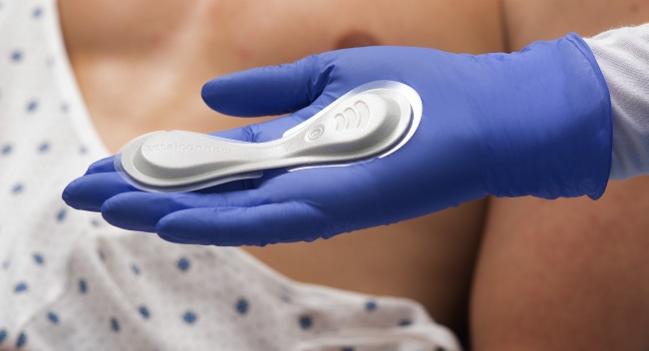
(UPDATED) An adhesive, wearable sensor the size and shape of a small sanitary napkin, in combination with machine learning, may give implantable devices a run for their money in forecasting heart failure exacerbations that lead to urgent hospitalizations.
The adhesive patch in combination with a continuous data collection platform that measured patient-level changes from baseline across a range of vital signs, accurately predicted unplanned hospitalizations a median of 6.5 days before hospitalization actually occurred.
Josef Stehlik, MD, MPH (VA Salt Lake City Health Care System, UT), and colleagues report the results of the LINK-HF study in an early online publication of Circulation: Heart Failure this week.
“I think it could be an attractive option, especially for patients where you can use it just during the high-risk period after a heart-failure hospitalization,” as was done in this study, Stehlik said.
“The advances in technology and advances in artificial intelligence are coming together so that we can have an approach that will work,” he explained, “and I think what is unique about this approach, too, is that you're not using absolute cutoffs for all patients. We establish a baseline for every patient and machine learning then tells us what is the expected behavior with these different physiological variables coming in. And when the observed deviates from the expected sufficiently, that is an indication that something is not right.”
Other invasive approaches have been trying to forecast heart-failure hospitalizations for years. Studies measuring intrathoracic impedance, multisensory data, and other cardiac resynchronization therapy (CRT)-based approaches have delivered mixed results. Other implanted devices like the CardioMems (Abbott) sensor, which measures pulmonary artery pressure, as well as other up-and-coming hemodynamic monitoring technologies like the V-LAP (Vectorious) have the disadvantage of being invasive, requiring right heart catheterization in the case of CardioMems and transseptal puncture in the case of the V-LAP.
Commenting on the study for TCTMD, Shelley Zieroth, MD (St. Boniface Hospital, Winnipeg, Canada), called the patch/continuous-monitoring combination used in LINK-HF “really interesting,” noting that it would be a particularly attractive solution for the large number of heart failure patients who do not already have an indication for CRT implantation, especially those with preserved ejection fraction (HFpEF), in whom CRT is not indicated.
“I think there's a good possibility that this would be competitive, in part because it's lower cost—that’s what's implied in the paper—it's noninvasive, and . . . it appears very user friendly,” she said.
The Missing Link
LINK-HF enrolled 100 patients with a mean age of 68 and NYHA II to IV functional class, all of whom were enrolled at the time of hospital discharge for an acute heart failure exacerbation. Each was fitted with the disposable sensor patch that includes a disposable battery with a 7-day charge and a reusable electronic module; the whole unit (Vital Connect) then adhered to the breastbone. The unit, including two skin-facing electrodes, records continuous ECG, continuous 3-axis accelerometry, skin impedance, skin temperature, heart rate, heart rate variability, arrhythmia burden, respiration, activity, sleep, body tilt, and body posture. All information was streamed to the wearer’s smartphone by Bluetooth and, in this study, was then encrypted and transmitted to a cloud-based analytics platform (pinpointIQ; physIQ), which uses proprietary analytics to generate each subject’s baseline physiological criteria and issues an alert when there are deviations.
When the observed deviates from the expected sufficiently, that is an indication that something is not right. Josef Stehlik
Importantly, explained Stehlik, machine learning derived from baseline data collection was used to establish “normal” for each study subject, such that perturbations in sensor-detected parameters were patient-specific. Also of note, this particular study was for data collection only: study physicians received alerts warning of perturbations, but they did not act on them in order to test the accuracy of the sensors in predicting hospital readmission due to worsening HF or any other reason for hospitalization.
Sure enough, 34 unplanned, nontrauma hospitalizations occurred during the 3-month follow-up, of which 24 were for worsening heart failure. The sensors/monitoring were able to pick up signals of pending hospitalization for HF exacerbation with a sensitivity that ranged from 76% to 88%, and with a specificity of 85%. The time between the initial alert and hospitalization ranged from 4.2 to 13.7 days.
According to Stehlik, that’s an ample window for physicians to potentially increase diuretic dose to prevent volume retention as well as to verify adherence to or uptitrate other guideline-directed medical therapy. He also noted that the algorithms could theoretically be altered depending on the risk level of the patient wearing the sensor, to increase either the specificity or sensitivity of the readings.
Twelve patients died during the LINK-HF study, and none of the deaths were predicted by the sensors/algorithm. To TCTMD, Stehlik stressed that numbers were small and that the technology would not likely be able to predict sudden cardiac deaths, which represented six of the deaths in the study. However, he added, “it's well known that every admission for heart failure means an increased risk of mortality for these patients, so it’s not just ending up in the hospital, it's also that the future carries a higher risk of mortality.” If a sensor could reduce hospitalizations, the hope would be that it would also cut down on deaths related to heart failure deterioration, he said.
Larger Study Planned
Investigators have applied for funding and hope to move ahead soon with a larger study to determine if changes to patient care triggered by an alert from the sensors and monitoring could reduce the number or duration of hospitalizations and improve patient quality of life.
Such a study, Zieroth noted, would ideally include a larger number of patients with HFpEF, who made up just 25% of this initial study, as well as more female patients. LINK-HF enrolled just two women.
“If you look at the diagram showing where the device is placed, there are anatomical concerns for women because of our breasts,” she said. “So, you’d want to make sure that this technology is equally applicable to women, and if it is, that's wonderful.”
To TCTMD, Stehlik acknowledged this limitation, pointing out that the study was conducted at four US Veterans Affairs (VA) hospitals, with funding from the VA. “We are hoping to enroll more women in our future trial,” Stehlik said. “Our hope is that this would work just as well in women and in younger patients, and [we] hope that future studies will show that.”
Another hurdle, Zieroth flagged, will be managing all the incoming data from these devices.
“I always get a little concerned about remote-monitoring technology and what the algorithm is on the receiving end for the physician. That's really the challenge,” she said. “Remote monitoring and having standardized response algorithm does require some infrastructure from the team, whether that be a nurse or a physician assistant to review those alerts and alarms. So if we could filter out the high-risk signals and even automate the standardized response algorithm, that would save the team a lot of time.”
Indeed, Stehlik anticipates a day when artificial intelligence and machine learning wouldn’t just be used to determine patient-level physiologic distress signals, but also used to directly inform the patient of the specific interventions required.
“When you are asking what type of medications would be checked or changed, artificial algorithms down the road might be able to provide that information straight to patients,” he said, “leaving the doctor out of the loop.”
Note: An earlier version of this story did not identify the proprietary analytics platform, misattributing the alerts to the sensor rather than to the continuous monitoring technology.
Photo Credit: Vital Connect
Shelley Wood was the Editor-in-Chief of TCTMD and the Editorial Director at the Cardiovascular Research Foundation (CRF) from October 2015…
Read Full BioSources
Stehlik J, Schmalfuss C, Bozkurt B, et al. Continuous wearable monitoring analytics predict heart failure hospitalization. The LINK-HF multicenter study. Circ Heart Fail. 2020;13:Epub ahead of print.
Disclosures
- LINK-HF was funded by the Department of Veterans Affairs Office of Information & Technology—the Veterans Affairs Industry Innovations (Vai2) Competition (VA118-11-P-0031) and by the Veterans Affairs Center for Innovation, Washington, DC.
- Stehlik reports funding from Abbott and Medtronic.
- Zieroth reports consulting for Abbott.


Comments