Two Large Studies Affirm Rare Thrombosis Risk With ChAdOx1 COVID-19 Vax
Researchers emphasized that the overall risk was small and needs to be balanced against the vaccine’s benefit.

Two new population-wide studies including millions of adults vaccinated against COVID-19 have confirmed earlier reports linking the ChAdOx1 vaccine (AstraZeneca/Oxford University) to an increased risk of intracranial thrombosis, as well as hospitalization for thrombocytopenia.
But as with the previous studies, the investigators emphasize that the risk is very small and needs to be balanced against the significant morbidity and mortality than can result from COVID-19.
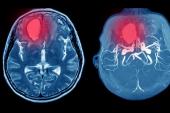
In the first study, which included 21 million adults vaccinated in England, rates of intracranial venous thrombosis (ICVT) and thrombocytopenia were higher in the 28 days after vaccination with the ChAdOx1 vaccine—but not BNT162b2 (Pfizer/BioNTech)—in younger adults when compared with the period prior to vaccination.
“Depending on your age and sex, between one and three people per million vaccinated were hospitalized with intracranial venous thrombosis, which is a very, very small number,” William N. Whiteley, BM BCh, PhD (University of Edinburgh, Scotland), who led the first cohort study, told TCTMD. “We saw no one who had it with the Pfizer product. The people who develop this complication are severely affected—and we shouldn’t say they aren’t because they are—but the complication is very rare.”
In the second study, which included a cohort of 11,637,157 people in England, Scotland, and Wales, Steven Kerr, PhD (University of Edinburgh), and colleagues also observed a twofold higher risk of cerebral venous sinus thrombosis (CVST) with ChAdOx1 in the first 28 days after vaccination, but no such risk was seen in people who received BNT162b2. Again, the risk was small, with investigators reporting that the vaccination added approximately 0.25 CVST events per million people vaccinated.
Both studies were published February 22, 2022, in PLoS Medicine.
‘Entire Population of England’
Several research groups have reported rare side effects with the recombinant adenovirus ChAdOx1 vaccine, leading regulatory agencies, including the European Medicines Agency to warn physicians of the possibility of thrombosis and thrombocytopenia after vaccination. The syndrome has been dubbed vaccine-induced thrombotic thrombocytopenia (VITT) and is believed to be an autoimmune response to platelet factor 4 in the absence of heparin, according to researchers. In these previous studies, thromboses were seen in unusual sites, such as cerebral venous sinuses or mesenteric or portal veins.
In May 2021, the British Joint Committee on Vaccinations and Immunization recommended not administering ChAdOx1 to adults 40 years and younger if an alternative vaccine was available. The World Health Organization also warns of a small risk of thrombosis and thrombocytopenia with the vaccine, but only recommends it not be used in those 18 years and younger. The AstraZeneca/Oxford vaccine is not approved for use in the United States.
Both vaccines are safe relative to the harm that could happen from an infection. William Whiteley
When the initial reports of VITT began to surface with ChAdOx1, Whiteley said they wanted to determine the risks of vaccination and turned to linked healthcare data from the British Heart Foundation Data Science Center.
“We had access to the entire population of England, and we knew quite a lot about them because we have their primary care records, and we knew when they had been vaccinated because the government has one database with all vaccine information,” he said. “We were then able to follow up on everybody from the time of vaccination to the date we did the study and to look at the incidence of all thrombotic complications. That’s important because the initial reports had been on intracranial venous thrombosis, or clots within the cerebral veins, and there was a worry there might be clots in other areas with concomitant clotting disorders, like stroke and heart attack.”
By the end of 2020, there were 46 million eligible adults registered with an English general practitioner. By March 2021, 46% received their first vaccination against COVID-19. The majority of people vaccinated during this time were white (79%) and younger than 70 years (84%).
On the whole, when compared with the period prior to vaccination, the adjusted hazards of venous and arterial thromboses at 1 to 28 days after ChAdOx1 vaccination were on par with what was observed with the BNT162b2 vaccine.
In people younger than 70 years, there was no risk of venous thrombosis in the first 28 days after the ChAdOx1 vaccine compared with before vaccination. In fact, the risk of venous thrombosis after ChAdOx1 vaccination was lower in the first 28 days in people ≥ 70 years (adjusted HR 0.58; 95%CI 0.53 to 0.63). Risks of arterial thrombosis, including the risks of MI and ischemic stroke, were also significantly lower after ChAdOx1 vaccination in the first 28 days in those < and ≥ 70 years. With the Pfizer/BioNTech vaccine, there was no excess risk of venous or arterial events in the first 28 days after vaccination, and like the ChAdOx1 vaccine, BNT162b2 appeared to be protective in older adults.
However, the risk of ICVT was more than twofold higher in adults < 70 years who received the AstraZeneca/Oxford vaccine (adjusted HR 2.27; 95% CI 1.33-3.88) compared with the prevaccination period. The risk of thrombocytopenia was also significantly higher (adjusted HR 1.71; 95% CI 0.66 to 4.49) in these younger people. There was no excess risk seen in those 70 years or older and the overall risks were similar in men and women. There was no excess risk of ICVT or thrombocytopenia in people who received the Pfizer vaccine regardless of age.
To TCTMD, Whiteley emphasized that data clearly show both ChAdOx1 and BNT162b2 substantially protect people against the risk of death and severe complications resulting from COVID-19.
“Both vaccines are safe relative to the harm that could happen from an infection,” said Whiteley. “In the UK, people under the age of 40 years are not recommended to have the AstraZeneca product—they’re all recommended to have the Pfizer vaccine or the Moderna product—and we only saw this complication in younger people.” For the older adults who received ChAdOx1, these results suggest protection against MI or stroke, he added, but he cautioned against overinterpreting these findings given the study’s observational nature.
Infrequent CVST Events
In the study by Kerr and colleagues, the researchers performed a self-controlled case series to compare CVST events in England, Scotland, and Wales prior to and after vaccination. Overall, there were 201 incident CVST events in the cohort between December 2020 and June 2021. The entire observation period consisted of a 90-day reference period prior to vaccination, a 2-week prerisk period directly prior to vaccination, and a 4-week observation period after receiving either the AstraZeneca or Pfizer vaccine.
Among the roughly 4.95 million people vaccinated with ChAdOx1, there were 27 incident cases of CVST during the 90-day reference period and 27 incident cases during the 28-day postvaccination period. Compared with the reference period, the rate of developing CVST in the first 28 days after vaccination with ChAdOx1 was approximately two times higher (incidence rate ratio 1.93; 95% CI 1.20 to 3.11). In contrast, there was no excess risk of CVST after vaccination with BNT162b2.
The researchers say their findings are consistent with other studies, including one from the European Medicines Agency. They point out that even though they had access to a large cohort, CVST events were infrequent and further corroborating studies are needed. Still, the new data “may be useful in risk-benefit evaluations for vaccine-related policies and in providing quantification of risks associated with vaccination to the general public,” write Kerr et al.
Michael O’Riordan is the Managing Editor for TCTMD. He completed his undergraduate degrees at Queen’s University in Kingston, ON, and…
Read Full BioSources
Kerr S, Joy M, Torabi F, et al. First dose of ChAdOx1 and BNT162b2 COVID-19 vaccinations and cerebral venous sinus thrombosis: a pooled self-controlled cases series study of 11.6 million individuals in England, Scotland, and Wales. PLoS Med. 2021;Epub ahead of print.
Whiteley WN, Ip S, Cooper JA, et al. Association of COVID-19 vaccines ChAdOx1 and BNT162b2 with major venous, arterial, or thrombocytopenic events: a population-based cohort study of 46 million adults in England. PLoS Med. 2021;Epub ahead of print.
Disclosures
- Whiteley reports serving on an advisory board for Bayer. Kerr reports no conflicts of interest.


Comments