Valve Fracture for ViV TAVI Linked With Higher Mortality
If fracture is needed to replace small surgical valves, it’s best to do it after the transcatheter device is in, registry data suggest.
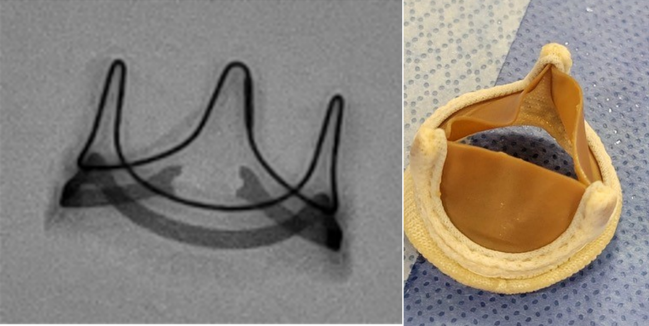
Photo Credit: Santiago Garcia
BOSTON, MA—Fracturing a surgical aortic valve to facilitate valve-in-valve (ViV) TAVI with the Sapien 3 or Ultra device (Edwards Lifesciences) is associated with a higher risk of in-hospital mortality, particularly if the old surgical valve is fractured before implanting the new transcatheter valve, according to data presented this week.
Compared with patients treated with ViV TAVI where the initial bioprosthetic valve wasn’t fractured as part of the procedure, those in whom fracture was done had a more than twofold higher risk of both all-cause mortality (OR 2.51; 95% CI 1.30-4.84) and cardiac mortality (OR 2.47; 95% CI 1.13-5.39), report investigators.
“We were surprised to see the safety signal at first,” lead investigator Santiago Garcia, MD (The Christ Hospital Health Network, Cincinnati, OH), told TCTMD. “When you step back and think about medicine, in general, you know that there is risk involved in any procedure. Quantifying that risk and understanding it is where we think we can move the ball forward. There is a role for this technique in patients who can’t have surgery that have small [prior] surgical valves and in whom we can manage to improve hemodynamics.”
Garcia, who presented the results during a late-breaking science session at TCT 2022, pointed out that despite the up-front mortality risk, there was only a small improvement in valve hemodynamics. The changes in echocardiographic outcomes were statistically significant, he said, but only “clinically modest.”
Higher Risk of Death, Modest Benefit
Garcia noted that there has been a tenfold increase from 2014 to 2019 in the number of ViV TAVIs done as an alternative to redo surgeries. Patient-prosthesis mismatch is an important limitation of the procedure, however, particularly in patients with small surgical valves, and remains an important predictor of long-term outcomes. Bioprosthetic valve fracture (BVF) can help expand the new transcatheter heart valve, improve mean gradients, and increase the effective orifice area.
For the new analysis, the researchers studied 2,975 patients who underwent ViV TAVI with the balloon-expandable Sapien 3 or Ultra device between late 2020 and early 2022 in the STS/ACC TVT Registry. Of these, BVF was performed in roughly one in five patients (n = 619), including 141 and 466 where the surgical valve was fractured before and after transcatheter valve implantation, respectively. Hemodynamic data are based on 1,085 patients with ViV TAVI for whom researchers knew the true internal diameter of the surgical valve.
In the adjusted analysis, fracturing the valve versus leaving it alone led to higher rates of death as well as higher rates of life-threatening bleeding (OR 2.55; 95% CI 1.44-4.50). There was also a trend toward more major vascular complications (OR 2.06; 95% CI 0.95-4.44). The mean aortic valve area at discharge was higher in those who had BVF versus those without (1.6 vs 1.4 cm2; P < 0.01) and the mean valvular gradient was lower (18.2 vs 22.0 mm Hg; P < 0.01).
When you step back and think about medicine, in general, you know that there is risk involved in any procedure. Santiago Garcia
When researchers looked at the timing of BVF, they found that fracture before TAVI was associated with a significantly higher risk of all-cause mortality (OR 2.90; 95% CI 1.21-6.94) and cardiac mortality (OR 3.42; 95% CI 1.25-9.37) versus the no-BVF patients. On the other hand, there was no statistical difference between patients who had the valve fractured after implantation. Life-threatening bleeding, major vascular complications, and new atrial fibrillation also were significantly higher if BVF occurred before the transcatheter implant. BVF also was associated with an increased risk of life-threatening bleeding when performed after TAVI.
Philippe Généreux, MD (Morristown Medical Center, NJ), who was not involved in the study, said the take-home message is that operators should only fracture the surgical valve after the transcatheter heart valve is in place. Despite the early hazards of BVF seen here, he emphasized that operators might not have a choice but to break the preexisting surgical valve. “If you have a gradient of 30 mm Hg, you don’t walk away, otherwise you’re going to have issues with valve durability,” he told TCTMD, noting high residual gradients can lead to valve thrombosis and early failure.
Given the clear difference in the pre- and post-implantation hazards, Garcia, like Généreux, said that if operators need to perform BVF, it’s safer and more efficacious to do so after the transcatheter valve is implanted. “Based on the information we have, when you have to do it, it’s usually in a small surgical valve with a true internal diameter of 21 mm or less,” he told TCTMD. “In patients that have high residual gradients after the transcatheter heart valve implant, that’s usually when you have to start thinking about this procedure.”
What’s not known, though, is the appropriate aortic valve gradient to move forward with BVF, Généreux said. “Should it be 15, 20 [mm Hg]? That’s what we need to better define.”
Thinking About the Future Today
Garcia noted that mortality rates were low in both groups, ranging from 1.0% to 1.5% in patients who didn’t have the prior valve fractured as compared with 2.5% to 3.0% in the BVF group. The study, he also stressed, is not designed to assess the clinical benefits of valve fracture. “The real question is whether the up-front risk is justifiable, if we’ll see a benefit at 1 year, 2 years, or 3 years. That remains unanswerable.”
In terms of technique, Garcia said most surgical valves are amenable to fracture but two are not because of their materials: Trifecta (St. Jude Medical) and Hancock II (Medtronic). The Magna and Magna Ease valves (Edwards Lifesciences), which have metallic stent frames, will fracture under higher balloon pressures, while the Mosaic valve (Medtronic) with its polymer coating will also fracture but at lower pressures.
Surgical valves are now being designed, he noted, with the prospect of future ViV TAVI. For example, the Inspiris Resilia aortic valve (Edwards Lifesciences) is designed with fluoroscopically visible size markers and an expandable cobalt-chromium alloy band to facilitate a ViV procedure should it be needed.
Overall, BVF with ViV TAVI remains a rare procedure. Of the 658 sites in the registry who performed ViV TAVI, 238 performed at least one BVF per year. On average, hospitals are doing just two BVFs each year, with only five sites doing 10 or more each year.
“Not everyone is doing it,” said Garcia. “It’s a low-volume procedure. We’re seeing all the ingredients where these patients should perhaps be sent to centers that do these routinely. It’s not something we do every day and that makes it hard to learn and protocolize.”
Michael O’Riordan is the Managing Editor for TCTMD. He completed his undergraduate degrees at Queen’s University in Kingston, ON, and…
Read Full BioSources
Garcia S, Bapat V, Depta JP, et al. Frequency and safety of bioprosthetic valve fracture in patients undergoing valve-in-valve TAVR for failed surgical valves using SAPIEN 3/Ultra valves: insights from real-world data. Presented at: TCT 2022. September 18, 2022. Boston, MA.
Disclosures
- Garcia reports consulting fees/honoraria from Boston Scientific, Edwards Lifesciences, and Medtronic.
- Généreux reports serving as a consultant to Abbott Vascular, Abiomed, BioTrace Medical, Boston Scientific, CARANX Medical, Cardiovascular Systems Inc, Edwards Lifesciences, GE Healthcare, iRhythm Technologies, Medtronic, Opsens, Pi-Cardia, Puzzle Medical, Saranas, Shockwave, Siemens, Soundbite Medical Inc, Teleflex, and 4C Medical; serving as an advisor to Abbott Vascular, Abiomed, BioTrace Medical, Edwards Lifesciences, and Medtronic; receiving speaker fees from Abbott Vascular, Abiomed, BioTrace Medical, Edwards Lifesciences, Medtronic, and Shockwave; and receiving an institutional research grant from Edwards Lifesciences, for which he also is a proctor. He is a principal investigator for trials by Cardiovascular Systems Inc, Edwards Lifesciences, and 4C Medical. He holds equity in Pi-Cardia, Puzzle Medical, Saranas, and Soundbite Medical.




Comments