Acurate Neo Fails to Match Results of CoreValve Evolut: SCOPE 2
Having failed noninferiority tests against two leading devices, Acurate neo’s niche is now in question, experts said.
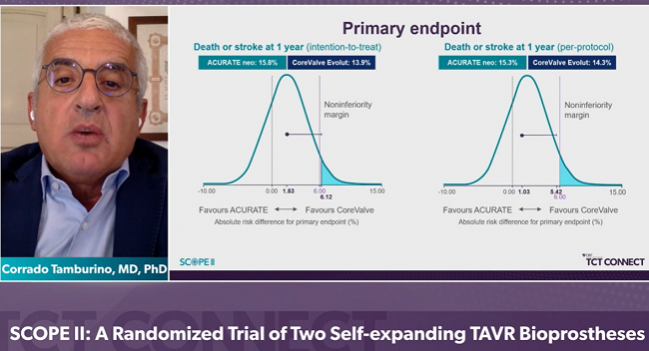
In a head-to-head study of two supra-annular, self-expanding TAVR devices, CoreValve Evolut (Medtronic) appears to be a better option than the Acurate neo valve (Boston Scientific) for the treatment of symptomatic severe aortic stenosis.
In SCOPE II, the primary endpoint of death or stroke at 1 year occurred in 15.8% of patients treated with Acurate neo and 13.9% of those who received CoreValve Evolut, a difference that failed to meet the trial’s statistical benchmark for noninferiority (P = 0.0549). Although there was no difference in all-cause mortality, rates of cardiac death, a secondary endpoint, were significantly higher Acurate neo.
Moderate or severe aortic regurgitation at 30 days also was significantly higher in patients who received the Acurate neo device (10.0% vs 3.0% with CoreValve Evolut; P = 0.002).
“Cardiac death is a secondary endpoint and as always it may suffer from lack of power and also misclassification in the absence of autopsy,” lead investigator Corrado Tamburino, MD, PhD (University of Catania, Italy), told TCTMD. “Although we cannot establish a conclusive link based on our data, a hypothesis is that there was more paravalvular leak with Acurate, and the link between paravalvular leak and cardiac death is well known in the literature. At 1 year, the most common cause of cardiac death in the study cohort was adjudicated by the clinical event committee as heart failure.”
This is the second time Acurate neo has come up short when compared to established TAVR systems. Last year, Acurate neo failed to establish noninferiority when compared with Sapien 3 (Edwards Lifesciences) in SCOPE 1.
Although there have been other randomized, head-to-head trials of different devices, such as CHOICE, SOLVE-TAVI, REPRISE III, and PORTICO IDE, Howard Herrmann, MD (Hospital of the University of Pennsylvania/Penn Medicine, Philadelphia), said SCOPE 2 is the first trial comparing two self-expanding transcatheter heart valves, even though Acurate neo is not available in the United States (it has been approved in Europe since 2014).
“So [SCOPE 2] has limited worldwide appeal, but what it showed was that Acurate neo was not as good as CoreValve Evolut,” said Herrmann, who wasn’t involved in the study. “Although Acurate neo has some advantages—it may protrude a little less into the ventricle and has less radial force, and that seems to lower the pacemaker rate compared with Evolut—it had more adverse effects that countered those.”
Herrmann also highlighted the 30-day SCOPE 1 data and noted that the 1-year data will be presented this Saturday during the late-breaking clinical science session at TCT Connect 2020.
Andrew Goldsweig, MD (University of Nebraska Medical Center, Omaha), who wasn’t involved in the study, called head-to-head trials “spotted zebras,” noting they are exceptionally rare. The major registries, including the Society of Thoracic Surgeons/American College of Cardiology TVT Registry, prohibit such trials and companies are reluctant to launch them given the cost and high financial stakes should their valve fail to come out on top.
“The structural heart disease community should take these rare head-to-head TAVR randomized controlled trials very seriously, because they represent the gold standard for comparing valves and we have so few of them,” said Goldsweig. Putting the numbers in perspective, he noted that TAVR is being performed in more than 250,000 patients each year, yet the six randomized head-to-head trials have included fewer than 4,000 patients, the largest being REPRISE III with 912 patients.
Taking on CoreValve Evolut
SCOPE 2 is a randomized, noninferiority study conducted at 23 high-volume centers in Europe. The study included 796 patients aged 75 years or older (mean 83.2 years; 68% women) with symptomatic severe aortic stenosis deemed at increased risk for mortality with surgical valve replacement (mean STS-PROM score 4.6%).
In addition to the intention-to-treat analysis, the researchers conducted a per-protocol analysis. In that comparison, Acurate neo was statistically noninferior to CoreValve with respect to the primary endpoint of death and stroke at 1 year (15.3% with Acurate neo vs 14.3% with CoreValve Evolut; P = 0.03 for noninferiority). However, as Tamburino pointed out in the press conference, the statistical plan did not allow them to claim noninferiority because the findings were inconsistent with the intention-to-treat analysis.
At 30 days, there was a significantly increased risk of cardiac death among patients treated with Acurate neo (3.0% vs 1.0% with CoreValve Evolut; P = 0.03), and this higher risk of cardiac death was still evident at 1 year (8.0% vs 4.0% with CoreValve Evolut; P = 0.01). As individual endpoints, there was no difference in the risk of all-cause mortality or stroke at 30 days or 1 year.
There was a bright spot, however, with the Acurate neo device associated with significantly less need for a new permanent pacemaker at 30 days (11.% vs 18.0% with CoreValve Evolut; P = 0.0027). In addition to a lower rate of new pacemaker implantation at 1 year, nearly all of which were implanted in the first 30 days, patients treated with Acurate neo had less new left bundle branch block.
To TCTMD, Tamburino said there are some theoretical advantages to the Acurate neo device, such as when a prosthesis with supra-annular design is desired or when there might be a need for future access to the coronary arteries in patients with CAD. The low pacemaker rate is also an upside. “However, these have to be weighed against the increase in paravalvular leak versus both [Sapien 3 and CoreValve Evolut] and the potential increase in cardiac death noted in SCOPE 2,” said Tamburino.
Herrmann said that permanent-pacemaker rates vary substantially, and while higher with CoreValve Evolut than with Acurate neo and Sapien, many experienced operators have “learned to implant Evolut higher using the cusp-overlap technique and have been able to lower their pacemaker rates to rates that are quite comparable to other devices.” At his center, pacemaker rates are similar with Evolut and Sapien devices, he said.
Boston Scientific recently launched the second-generation device, known as Acurate neo2, to selected European centers. The device has a new annular sealing technology that can better fit irregular, calcified anatomies and prevent paravalvular regurgitation, according to the company. Both Herrmann and Tamburino suggested that if the sealing technology can reduce the rate of aortic regurgitation, it may influence mortality at 30-days and 1-year.
“But I think we would need some of that data before we would want to adopt it on faith that it is going to be better than Acurate neo,” said Herrmann.
Understanding Niches and Nuances
Taking a big picture approach, Goldsweig speculated that given the results from SCOPE 1 and 2, Boston Scientific might consider more specific indications where Acurate neo might be useful. These specific subsets might include, for example, bicuspid or heavily calcified valves or even native valves with eccentric annuli. “Despite the SCOPE 1 and SCOPE 2 results, which are obviously disappointing, I don’t think we’ve seen the last of Acurate neo,” he said.
As an example, Goldsweig noted there are signals from observational studies suggesting Sapien 3 is superior to CoreValve Evolut with respect to all-cause mortality, and Medtronic recently launched the SMART trial that will compare CoreValve Evolut PRO/PRO+ and Sapien 3/Ultra in patients with small annuli.
“SMART will allow Medtronic to look for a more niche indication for their valve if it ultimately has higher mortality than Sapien in all-comers,” said Goldsweig. Asked if he could imagine the second-generation Acurate neo2 succeeding in an all-comer population, Goldsweig said you’d expect to see more promising signs than what was seen in SCOPE 1 and SCOPE 2.
As the primary SMART investigator, Herrmann said the trial is testing the two leading transcatheter heart valves in a broad population of patients, including younger patients, those at low risk for surgery, patients with bicuspid valves, patients with left ventricular dysfunction, and those undergoing TAVR-in-SAVR. The primary endpoint is noninferiority with respect to all-cause mortality, stroke, or rehospitalization at 12 months and superiority of CoreValve Evolut over Sapien 3 with respect to hemodynamic performance. It’s expected the trial will enroll a large percentage of women given the small annuli entry criteria, said Herrmann.
“The future is going to be understanding the nuances of these devices and so that we can tailor clinical decision-making to choose the best valve for each individual,” he said.
Michael O’Riordan is the Managing Editor for TCTMD. He completed his undergraduate degrees at Queen’s University in Kingston, ON, and…
Read Full BioSources
Capodanno D, Tamburino C, Bleiziffer S, et al. Comparison of self-expanding bioprostheses for transcatheter aortic valve replacement in patients with symptomatic severe aortic stenosis: the SCOPE 2 randomized clinical trial. Circulation. 2020;Epub ahead of print.
Disclosures
- Capodanno reports speaker and/or consulting fees from Abbott Vascular, Amgen, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Menarini, and Sanofi.
- Herrmann reports institutional grant support from Abbott Vascular, Ancora, Bayer, Boston Scientific, Edwards Lifesciences, Medtronic, Shockwave, University of Laval, and W.L. Gore & Associates; personal fees/honoraria from Edwards Lifesciences, Medtronic, and Shockwave; and equity/stock/options from Microinterventional Devices and Holistick.


Comments