APOLLO: Novel siRNA Injectable Cuts Lp(a) by Nearly 100% in Small Study
Lp(a), called the holy grail of prevention research, was reduced dramatically with SLN360 and should spur further research.
WASHINGTON, DC—Gene-silencing therapy can significantly reduce the production of lipoprotein(a), a hot target in the field of atherosclerotic cardiovascular disease (ASCVD) prevention, early data suggest.

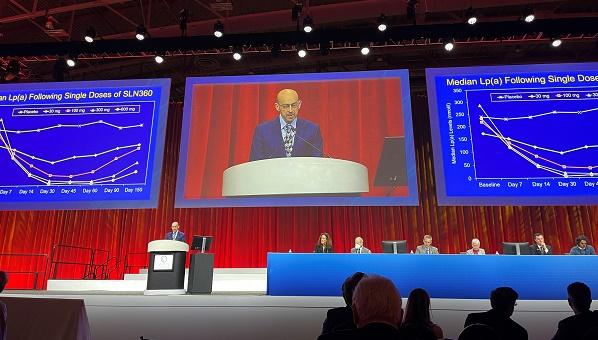
Among individuals treated with the highest dose of a small-interfering RNA (siRNA), the single, subcutaneous injection reduced Lp(a) levels by as much as 98%, reported Steven Nissen, MD (Cleveland Clinic, OH), during the late-breaking clinical trial session at the American College of Cardiology 2022 Scientific Sessions.
“Historically, lipoprotein(a) has been considered an untreatable abnormality, and it’s frequently not measured by many of our colleagues,” said Nissen. “The development of therapies targeting mRNA has made it possible to significantly lower Lp(a)—whether these reductions can impact the incidence of ASCVD or prevent the progression of aortic stenosis remains to be determined, but we think that optimism is warranted.”
Historically, lipoprotein(a) has been considered an untreatable abnormality and it’s frequently not measured by many of our colleagues. Steven Nissen
Vera Bittner, MD (University of Alabama at Birmingham), who commented on the study during the session, said the development of agents designed to specifically Lp(a) has been considered the “holy grail” of ASCVD prevention for several years. She pointed out that previous epidemiologic studies have suggested that these drugs will need to robustly lower Lp(a) levels to impact ASCVD or aortic stenosis event rates. For that reason, APOLLO can be considered a successful phase I study of an mRNA with sufficiently potent Lp(a)-lowering.
Michael Blaha, MD (Johns Hopkins University Medical Center, Baltimore, MD), too, was impressed by the data, saying the field’s focus on Lp(a) is extremely exciting. “This is something that we know explains quite a bit of the residual risk that we see in patients and the unexplained familial risk that we can’t do anything about,” he told TCTMD. “I love that we’re seeing multiple different approaches to lowering Lp(a).”
Like Bittner, Blaha noted that the epidemiology data suggest that treatment will require a very robust reduction in Lp(a) to be beneficial. “And we’re seeing that,” he said.
The study was published simultaneously April 3, 2022, in JAMA.
Other Agents in Development, Too
SLN360 (Silence Therapeutics), as the siRNA is currently known, targets LPA, which is the gene that encodes for apolipoprotein(a), the “dominant, rate-limiting component” in the liver’s production of Lp(a). The siRNA degrades the mRNA of apolipoprotein(a), which in turn reduces Lp(a). Pelacarsen (Novartis), which is another Lp(a)-lowering therapeutic currently in clinical testing, is an antisense oligonucleotide that also targets the mRNA of LPA.
Lp(a) is known to be associated with CVD, both in primary- and secondary-prevention settings, as well in mendelian randomization studies. In the US guidelines for the treatment of high cholesterol, elevated Lp(a) is considered a risk-enhancing factor to help physicians and patients make an informed decision about LDL cholesterol-lowering treatment. In Europe, the clinical guidelines recommend checking Lp(a) for CVD risk stratification in individuals at least once during the patient’s lifetime, although the recommendation is relatively weak.
I love that we’re seeing multiple different approaches to lowering Lp(a). Michael Blaha
In the APOLLO trial, investigators randomized 32 patients in four cohorts to different SLN360 doses—each cohort contained six patients randomized to active treatment and two participants treated with placebo. Across the four cohorts, patients were treated with 30 mg, 100 mg, 300 mg, and 600 mg of SLN360, respectively. None of the participants had preexisting cardiovascular disease, but all had Lp(a) concentrations ≥ 150 nmol/L at baseline, which is approximately 60 mg/dL.
The maximal median reduction in Lp(a) was 20, 89, 185, 268, and 227 nmol/L for those treated with placebo and the 30, 100, 300, and 600 mg doses, respectively. The maximal median percentage reduction from baseline was 10%, 46%, 86%, 96%, and 98%, respectively. The nadir of Lp(a) reduction occurred between 30 and 60 days after treatment with all doses. After this point, Lp(a) values rose again, but they did not return to baseline values by 150 days. For the 300- and 600-mg doses, Lp(a) levels remained roughly 70% and 80% lower from baseline at 150 days.
At the maximal SLN360 doses, LDL cholesterol and apolipoprotein B were reduced 26% and 24%, respectively.
In terms of safety, headache was reported by nine participants, including five treated with the 600-mg dose. Neutrophil counts increased in three patients, as did C-reactive protein levels. There was one serious adverse event—a patient receiving the 30-mg dose hospitalized for fever and severe headache—but those effects were ascribed to the COVID-19 vaccine they’d received a week earlier. The same patient had a threefold elevation in liver enzymes, and again this was chalked up to the COVID-19 vaccine. Overall, treatment-emergent adverse events were mild and were most commonly low-grade injection site events. None resulted in study withdrawal.
Researchers stress, however, that the present study was too small to truly determine the safety of this siRNA therapeutic and further studies are needed, including those with a more-diverse patient population, such as those with cardiovascular disease.
Anthony DeMaria, MD (UC San Diego Health, La Jolla, CA), who commented on the study, listed its shortcomings first, namely that it was a small, single-center study using a surrogate endpoint. Nonetheless, he imagines the game changing fairly soon. “In the past, I would say that every adult should know at least two numbers: their blood pressure and their cholesterol,” he said. In the future, knowing your LDL and HDL levels won’t be enough, though. “People have not been accustomed to measuring Lp(a) levels,” DeMaria said, “but I think we have to start to do that because we’re going to need to identify patients who are at risk.”
First steps first, said Blaha, is to determine whether lowering Lp(a) lowers ASCVD event rates. Pelacarsen, the antisense oligonucleotide further along in clinical development, is currently being tested in the large-scale Lp(a)HORIZON study, of which Nissen is serving as the study chairman. That said, Blaha is also optimistic about the future for patients with elevated Lp(a).
“The data is sort of lining up just perfectly,” he said. “In fact, if the [CVD event] trials aren’t positive, I think there’ll be a lot of head-scratching about how’s that’s possible. Of course, you never know, though.”
More Data Coming
Speaking with the media, Nissen said that patients with elevated Lp(a) levels are frequently encountered in the cardiac care unit after a myocardial infarction. While Lp(a) is a powerful risk factor for ASCVD, it’s only considered a risk-enhancing factor right now and is not often on the radar of physicians. Like DeMaria, Nissen believes that at least once in a person’s lifetime, preferably when they’re young, Lp(a) should be measured. At their prevention clinic, all patients undergo Lp(a) testing, he said.
“As these therapies mature, we’re going to have to know who to treat,” said Nissen.
When patients with high Lp(a) are identified today, Nissen said, the goal should be to treat the other risk factors as aggressively as possible. That includes lowering LDL cholesterol as low as possible, getting blood pressure under control, and getting overweight/obese patients physically active. “We’ll take every other risk factor off the table until we have drugs on the market that can treat Lp(a)—that’s why we need to measure it,” he told TCTMD.
Blaha takes a similar approach when he discovers a patient has significantly elevated Lp(a), such as those with levels exceeding 150 nmol/L. “I’ll counsel patients on the fact that they have inherited familial risk that we can’t do much about right now so we need to lower their other risk factors even more,” he said. “I also counsel them on cascade screening, particularly if they have adult children who also should be screened, especially if they have a strong family history.”
Finally, Blaha said that he’ll counsel patients about potentially enrolling in a clinical trial, noting his center is enrolling patients in Lp(a)HORIZON, although that is a secondary-prevention trial. If they’re ineligible, he’ll tell them that it’s possible there’ll one day be a therapy to treat their elevated Lp(a) levels.
During the discussion, Bittner said that in past studies of the PCSK9 inhibitors—which also have been demonstrated to lower Lp(a) levels—the largest reduction in Lp(a) was seen among individuals with highest baseline levels. Nissen said they were unable to analyze the reduction in Lp(a) based on starting Lp(a), but that it’s an interesting question. However, because the therapy is so effective at degrading mRNA of LPA, “it’s quite likely we’ll see robust suppression of plasma [Lp(a)] levels regardless of the baseline level—that’s unproven, but I think it’s very likely,” Nissen predicted.
Michael O’Riordan is the Managing Editor for TCTMD. He completed his undergraduate degrees at Queen’s University in Kingston, ON, and…
Read Full BioSources
Nissen SE, Wolski K, Balog C, et al. Single ascending dose study of a short interfering RNA targeting lipoprotein(a) production in individuals with elevated plasma lipoprotein(a) levels. JAMA. 2022;Epub ahead of print.
Disclosures
- Nissen reports grant support from Novartis. He also reports conducting clinical trials funded by AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Eli Lilly, Esperion, Medtronic, Novartis, Silence Therapeutics, and Pfizer.




Comments