AXIOMATIC-TKR: Milvexian Promising for VTE Prevention
If confirmed in larger populations, factor XIa inhibitors could be a valuable oral alternative to LMWH, says Geoffrey Barnes.
The oral factor XIa inhibitor milvexian is a promising option for preventing venous thromboembolism (VTE) while keeping bleeding risk low, according to the randomized AXIOMATIC-TKR trial.
Presented in a late-breaking science session at the virtual American Heart Association 2021 Scientific Sessions and published simultaneously in the New England Journal of Medicine, AXIOMATIC-TKR was a phase II trial of patients undergoing elective knee arthroplasty who were randomized to oral milvexian or injectable enoxaparin.
Speaking with TCTMD, lead author Jeffrey I. Weitz, MD (McMaster University, Hamilton, Canada), said although there was a significant dose-response for efficacy with both twice-daily and once-daily milvexian (Bristol-Myers Squibb/Janssen Pharmaceuticals) across a range of doses, from 25 mg to 400 milligrams per day, there was no apparent dose-dependent increase in bleeding.
“This speaks to the potential wide therapeutic window of an oral factor XIa inhibitor like milvexian,” Weitz said. He added that there is increasing interest in factor XI as a target for new anticoagulants due to mounting evidence that it is important for thrombosis, but “mostly dispensable” for hemostasis.
“Therefore, targeting factor XI has the potential to be safer than targeting downstream clotting enzymes like factor Xa or thrombin,” Weitz said.
Geoffrey Barnes, MD (University of Michigan, Ann Arbor), who was not involved in the study, said that although milvexian and the factor XIa inhibitors as a class are still under development, the AXIOMATIC-TKR results are an important step toward understanding what they can do.
“We've got some really exciting data from an oral agent in a new therapeutic target that shows some promising efficacy and safety,” said Barnes, who is a member of the American College of Cardiology’s Peripheral Vascular Disease Council. “But it's going to have to be borne out in a much larger phase III study before it would be ready for clinical use.”
No Increased Bleeding
In their paper, Weitz and colleagues note that patients undergoing elective knee arthroplasty are a frequent starting point for the development and dose-finding of new anticoagulants. This is because efficacy can be objectively assessed with venography for presence of deep-vein thrombosis (DVT) after surgery.
Barnes said while many patients only need aspirin after these surgeries, options for those needing optimal VTE prevention are limited to low-molecular-weight heparin (LMWH) or factor Xa inhibitors, both of which have higher efficacy than aspirin, but also higher bleeding risk. Looking longer-term, he said AXIOMATIC-TKR provides good efficacy data on milvexian, but “a more diverse population will really help us get at some more of the needed safety data.”
AXIOMATIC-TKR enrolled 1,242 knee arthroplasty patients aged 50 or older from 118 centers in 18 countries. They were randomized postoperatively to either a 40-mg injection of enoxaparin once daily or one of seven milvexian dose regimens(25 mg, 50 mg, 100 mg, or 200 mg twice daily or 25 mg, 50 mg, or 200 mg once daily). Treatment adherence was assessed by pill counts or enoxaparin syringe counts. Three-quarters of the study population were 100% compliant with their assigned regimen. Mandatory venography was performed 10 to 14 days after the surgery date.
If we could move beyond the low-molecular-weight heparin parenteral need and have a really effective and safe oral option, that would be potentially beneficial. Geoffrey Barnes
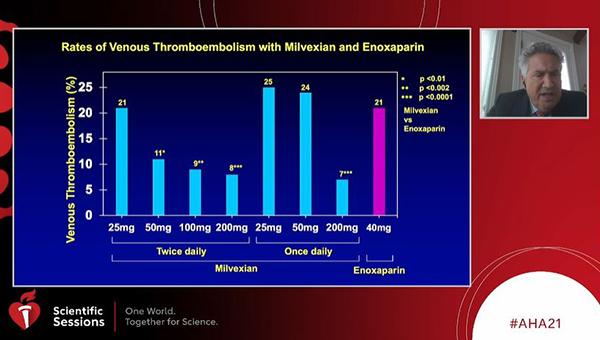
The primary efficacy outcome of VTE (a composite of asymptomatic deep-vein thrombosis, confirmed symptomatic VTE, or death from any cause) in the milvexian twice-daily groups occurred in 21% of patients on 25 mg, 11% on 50 mg, 9% on 100 mg, and 8% on 200 mg. The dose-response relationship was also seen in the once-daily milvexian groups, with a rate of VTE of 25% in those on 25 mg, 24% in those on 50 mg, and 7% in those on 200 mg. In all groups except the 25-mg group, the dose-response relationships for twice-daily milvexian were significant for occurrence of VTE in comparison with enoxaparin: P < 0.01 for 50 mg, P < 0.002 for 100 mg, and P < 0.0001 for 200 mg. The 200-mg once-daily milvexian dose also was significant at P < 0.0001.
In the milvexian twice-daily group as a whole, VTE occurred in 12%, coming in well below the prespecified benchmark of 30% (one-sided P < 0.001). In comparison, the rate of VTE among the 252 patients who took enoxaparin was 21%. Additionally, three patients had symptomatic nonfatal pulmonary embolism (one assigned to enoxaparin group and two in the milvexian groups).
Bleeding of any severity occurred at a rate of 4% in both the enoxaparin and combined milvexian groups, with no major bleeding episodes in the milvexian patients and one in the enoxaparin group. Clinically relevant bleeding, a composite of major bleeding and clinically relevant nonmajor bleeding occurred in 1% of milvexian patients and in 2% of enoxaparin patients. No dose-response relationship was seen with regard to bleeding. Serious adverse events were reported in 2% of the milvexian group and 4% of the enoxaparin group. To TCTMD, Weitz said these were unrelated to the drug itself and consisted of incidents where investigators suspected pulmonary embolism or DVT but didn’t necessarily confirm those suspicions with testing.
Future Impact
According to Barnes, if further research can confirm and extend the AXIOMATIC-TKR results, there could be additional positive impacts of milvexian on patient care.
“We know that when patients are given a pill option rather than a parenteral option, they're much more likely to be compliant. So, that's a real plus for this drug,” he observed. “If we could move beyond the low-molecular-weight heparin parenteral need and have a really effective and safe oral option, that would be potentially beneficial.” In addition to milvexian, he said, a number of other factor XIa inhibitors are currently in development, including testing in phase II studies.
“I do think we're going to see a number of these being explored further in the next few years,” Barnes said. “Obviously, the hope for patients is that we can find a few that are easy to take, that are as efficacious or more efficacious than our current treatments, and that potentially could reduce the risks of bleeding. That would really be a game changer if it all comes together.”
Weitz said that based on the AXIOMATIC-TKR dose-finding efforts, “the magic number is at least 100 milligrams per day,” for milvexian, given as a once- or twice-daily regimen. He further noted that milvexian is currently undergoing phase II evaluation for secondary stroke prevention in the AXIOMATIC-SSP study, where it will be added to aspirin and clopidogrel.
“That study will give safety information on the use of milvexian in conjunction with dual and with single antiplatelet therapy. That information is needed, together with this study, to identify the optimal doses that can be carried forward into phase III programs,” he concluded.
Regarding reversibility of milvexian, Weitz said during a discussion after his presentation that there is no specific reversal agent for the drug, which has a half-life of about 12 hours.
“What we're finding with factor XI inhibitors is that if you do run into trouble, you can use tranexamic acid, an antifibrinolytic agent, to manage bleeding,” he said, adding that in patients with congenital factor XI deficiency, what is being used successfully is very low doses of recombinant factor VIIa to bypass the factor XI deficiency or inhibition.”
L.A. McKeown is a Senior Medical Journalist for TCTMD, the Section Editor of CV Team Forum, and Senior Medical…
Read Full BioSources
Weitz JI, Strony J, Ageno W, et al. Milvexian for the prevention of venous thromboembolism. N Engl J Med. 2021;Epub ahead of print.
Disclosures
- Weitz reports consulting for Alnylam, Anthos, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Pfizer, Ionis, Janssen, Merck, and Regeneron; and serving on scientific advisory boards of Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Pfizer, Portola, Ionis, Janssen, and Servier.




Comments