Bentracimab Rapidly, Safely Reverses Ticagrelor in Early REVERSE-IT Data
The interim results mostly center around patients needing urgent surgery but are significant for bleeding patients as well.
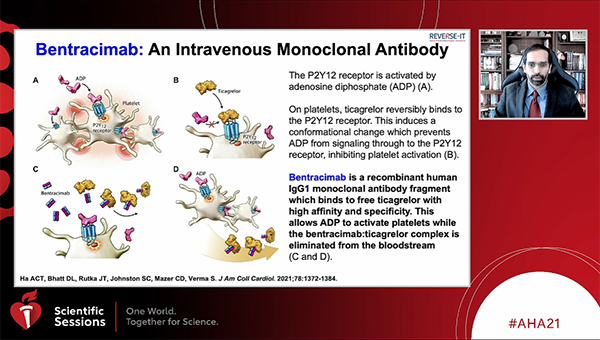
Bentracimab (PhaseBio), an intravenous reversal agent for ticagrelor, provides immediate and sustained reversal of the P2Y12 inhibitor’s antiplatelet effects in both patients undergoing surgery and those with major bleeding, according to a prespecified interim analysis of the REVERSE-IT study.
Further, effective hemostasis was graded good or excellent in the vast majority of cases, and there were no drug-related serious adverse events or allergic reactions.
The drug is a recombinant monoclonal antibody fragment that binds to both ticagrelor and its major active circulating metabolite, reversing the antiplatelet effects within 5 minutes. Previous phase I data in healthy participants, published in 2019, sparked excitement as a potential answer to the challenges faced not just by surgeons, but also by physicians when patients on ticagrelor present with an intracranial hemorrhage, for example, and can’t wait the recommended 5 days for the drug’s effects to wear off.
Deepak L. Bhatt, MD, MPH (Brigham and Women’s Hospital, Boston, MA), who presented the data today during a late-breaking trial session at the virtual American Heart Association 2021 Scientific Sessions, told TCTMD the reasoning behind this prespecified interim analysis “is with the hope of an accelerated, although conditional, approval” of the drug by US and European regulatory agencies. Bhatt said he would also love to see the drug approved for compassionate use at this time, as he receives weekly call from physicians around the country wanting to use the investigational drug for dire cases.
Reversal agents for non-vitamin K antagonist oral anticoagulants (NOACs) like andexanet alfa (Andexxa; Alexion) “have been really a big advance for patients with bad bleeding and the hope is now for ticagrelor there would be something like it,” Bhatt continued. “Although my hope is that it will be priced in a way that is much more favorable than the NOAC reversal agents, which are quite expensive and that's limited their use.”
Based on two recent label expansions for ticagrelor for high-risk CAD primary prevention as well as stroke prevention and its upcoming patent expiration date, Bhatt predicts ticagrelor’s use will grow. “And if there is a ticagrelor reversal agent, I think it makes ticagrelor use even more appealing versus potentially clopidogrel or prasugrel, especially once all three are generic,” he said. “If you can reverse something and you can do it pretty easily and it doesn't cost too, too much to do that reversal, then it does provide an advantage for that particular antiplatelet agent.”
Interim Results
For the study so far, Bhatt and colleagues have treated 150 patients with bentracimab—142 with planned urgent surgery (92% cardiac surgery) and eight with major bleeding. Mean patient age was 65 years and 77.3% were male, with 71.3% of patients within 1 day from their last ticagrelor dose.
On both the VerifyNow platelet function assay and VASP platelet reactivity index, platelet reactivity was shown to be significantly reversed within 4 hours of bentracimab initiation as well as at all time points through 24 hours (P < 0.0001). This was consistent in both surgical and bleeding patients as well as in all prespecified subgroups.
Among surgical patients, adjudicated hemostasis was achieved in all, with a 39% rate of total blood transfusions. For bleeding patients, excellent or good hemostasis was adjudicated in 77.8% with a total blood transfusion rate of 62.5%. The median time to restart P2Y12 inhibition for surgical and bleeding patients, respectively, was 2 and 5 days.
Notably, there was no platelet rebound activity within 72 hours. There were eight reported thrombotic events—none were related to bentracimab—and no allergic reactions seen.
Given the low prevalence of bleeding patients in the trial so far, Bhatt said his team’s goal is to focus on this group for continued enrollment. “So there will be more data than just the bleeding patients that we have here in real life,” he said.
Previous discussion around the drug centered on its potential need to be titratable, although Bhatt said this has not yet been analyzed. “We studied it in more of a fixed way, but in the future one could envision a situation where if you wanted partial reversibility you could do that,” he said. “If you wanted reversal for 2 days because someone has an intracranial hemorrhage you could do that. If you wanted reversal for an hour because you were going to do a pericardiocentesis for someone on ticagrelor you could do that.”
To keep things simple, Bhatt said he would like to see the drug approved in the way it was studied in REVERSE-IT, “but certainly down the road one could easily conceive of a way of titrating it.”
Commenting on the study for TCTMD, B. Hadley Wilson, MD (The Sanger Heart & Vascular Institute/Atrium Health, Charlotte, NC), vice president-elect for the American College of Cardiology, said “this is one of the more exciting presentations” to come out of the AHA meeting.
He envisions bentracimab being employed similar to the way it was done in REVERSE-IT, “which [means] it's going to be probably used a lot more frequently for those that need to undergo urgent surgery,” he said. “Although I think it's also going to be very important for those that have major bleeding problems and need reversal of ticagrelor as well.”
Notably, the population who could benefit most from bentracimab is different from those who might be able to wait 2 or 3 days, as was recently deemed safe in RAPID CABG, for ticagrelor’s effects to wear off, Wilson said. “I think that's an important difference to make, which is that in this study patients were able to undergo coronary bypass surgery within 24 hours. So there are patients that have critical coronary anatomy like left main or stent reocclusion and other multivessel disease problems . . . and this would be a great option or maybe even a lifesaving option for these patients.”
Because of the low side-effect profile, he also said he would “like to see it move along to see if it can be used in compassionate use cases in the near future.” The availability of a reversal agent, once approved, “would actually increase the use of ticagrelor in [dual antiplatelet therapy] knowing that there is a way to reverse it,” Wilson added.
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Bhatt DL. REVERSE-IT: effect of bentracimab on platelet inhibition and hemostasis in patients on ticagrelor with major bleeding or requiring urgent procedures. Presented at: AHA 2021. November 15, 2021.
Disclosures
- The study was funded by PhaseBio.
- Bhatt reports receiving research funding or unfunded research support from Abbott, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi, Synaptic, The Medicines Company, FlowCo, Merck, Novo Nordisk, PLx Pharma, and Takeda; being a site co-investigator for Biotronik, Boston Scientific, St. Jude Medical, and Svelte; being a trustee for ACC; serving as an advisory board member, director, or chair for Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; the Boston VA Research Institute, the Society of Cardiovascular Patient Care, TobeSoft; the American Heart Association Quality Oversight Committee; serving on a range of data safety monitoring committees; receiving honoraria for editorial or committee activities for a range of publications and organizations; and receiving royalties from Elsevier.
- Wilson reports no relevant conflicts of interest.





Comments