Bioresorbable PFO/ASD Closure Device Shows Promise, but More Research Needed
Potential candidates are patients with nitinol allergies and those who might need future left heart procedures, researchers say.
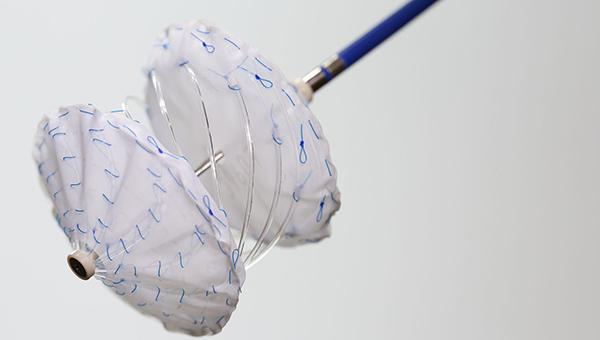
Initial data looking at safety and efficacy of an interatrial septal occluder with a bioresorbable frame show promise for closing atrial septal defects (ASDs), but the study authors suggest a redesign for the device to be used specifically in patent foramen ovale (PFO) due to less-than-stellar outcomes there.
After years of negative or equivocal clinical trials plagued by slow enrollment and polarized opinions, a number of transcatheter devices are now available and US Food and Drug Administration approved for ASD and PFO closure. However, all to date have durable nitinol frames. This can pose a problem for the small proportion of patients who might be allergic to metal alloys, and questions remain as to the long-term safety of these devices over decades, given that many of the people who get them are young adults at the time.
Even with the success seen with closure so far, senior author of the study Horst Sievert, MD (CardioVascular Center Frankfurt CVC, Germany), told TCTMD, “there is nothing in the world which is good enough to leave alone and not improve.” Compared with a procedure like TAVI, for example, PFO/ASD closure is “prophylactic”—intended for stroke prevention—and as such should be associated with “zero complications long-term,” he said, adding that he has seen clots form on PFO devices up to 12 years after implant as well as erosions up to 15 years later. Though these problems are rare and statistically irrelevant, Sievert said, “if you are that patient, you will suffer, so it's important to avoid these complications.”
Complications like these “cannot happen,” in theory, with a bioresorbable device that is eventually replaced by tissue, he observed.
That said, bioresorbable devices come with their own sets of hurdles—bioresorbable stents, for example, have failed so far to pan out in the setting of coronary disease after early trials suggested that the scaffolds actually increased, rather than reduced, rates of stent thrombosis.
On and Off the Market
The Carag bioresorbable septal occluder (CARAG AG) received CE Mark in 2017 but is no longer commercially available in Europe due to new evidence requirements by EU regulatory authorities, lead author Kolja Sievert, MD (CardioVascular Center Frankfurt CVC, Germany), told TCTMD. The device has since been rebranded as the reSept ASD occluder (atHeart Medical AG) and is undergoing further study in a multicenter US-based trial of 250 patients.
For this analysis, published online ahead of print in EuroIntervention, the researchers included nine patients with ASD and six with PFO treated with the Carag occluder at their institution between 2014 and 2016. Overall procedural success was 88.2%, with an average ASD size of 16.5 mm and an average PFO diameter of 8.0 mm. Poor septal alignment was the cause of two failed implantations, one in a PFO and one in a patient with an ASD.
Over 24-month follow-up, clinically-effective closure was seen in 100% of ASDs but in only 50% of PFOs, for which one large and two moderate residual shunts were observed. Notably, transesophageal echocardiography confirmed no device deformation at this time point. Also, the only device-related serious adverse events observed were palpitations and thrombus in one patient each, with the latter being resolved by anticoagulation.
“Compared to the results reported for other PFO devices, these results are suboptimal, however, the number of patients is too small to draw any conclusions,” the authors write. “Neither tunnel length nor PFO size correlated with the closure rate. The compliance of septum primum may play a more important role with this device and perhaps using a larger device size may have led to better outcomes.”
Next Steps
While the goal of “leaving nothing behind” would be important for all patients, it would prove especially advantageous for those needing procedures down the line that require left-atrial access, Horst Sievert said. “Very often we can puncture below the metallic devices or even through the metallic devices, but to have something which is bioresorbable helps to do, let's say, MitraClip 20 years later after device closure of an ASD.”
“We learned through these initial cases that the device is not so good for PFOs with a long tunnel,” Horst Sievert said. “So the current design is really for ADSs or for PFOs with very short tunnel. . . . Most PFOs have a tunnel, so I think it has to be a little bit different design.”
Still, closure rates, “could have been worse,” he said, calling the results as “very good” for a first attempt at closure with a bioresorbable device.
Commenting on the study for TCTMD, Steven R. Messé, MD (University of Pennsylvania, Philadelphia), was less sanguine, noting in an email that the results were “underwhelming for the PFO patients” and that the small size of the study cannot allow for firm conclusions. That said, he expects that “more devices and approaches to PFO closure will be coming via the less-onerous 510(k) process. This regulatory pathway allows for approval of devices that are substantially equivalent in efficacy and safety to previously approved predicate devices and generally is considered less stringent.”
He welcomes “anyone to come up with a better, safer means” to perform closure and suggested that a bioresorbable device “may be advantageous,” so long as the safety and efficacy are comparable to currently approved devices.
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Sievert K, Bertog S, Söderberg B, et al. Transcatheter closure of atrial septal defect and patent foramen ovale with Carag bioresorbable septal occluder: first-in-man experience with 24-month follow-up. EuroIntervention: 2021;Epub ahead of print.
Disclosures
- The study was sponsored and funded by CARAG AG.
- Horst Sievert reports receiving fees from 4tech Cardio, Abbott, Ablative Solutions, Ancora Heart, Bavaria Medizin Technologie GmbH, BioVentrix, Boston Scientific, Carag, Cardiac Dimensions, CeloNova, Cibiem, CGuard, Comed B.V., Contego, CVRx, Edwards, Endologix, Hemoteq, InspireMD, Lifetech, Maquet Getinge Group, Medtronic, Mitralign, Nuomao Medtech, Occlutech, pfm medical, ReCor Medical, RenalGuard, ROX Medical, Terumo, Vascular Dynamics, Vivasure Medical, Venus Medtech, and Veryan.
- Kolja Sievert reports no relevant conflicts of interest.
- Messé reports serving as a PI for the Gore REDUCE PFO closure trial and a sub-investigator for the CLOSURE I PFO closure trial as well as having research grant funding from Gore to evaluate outcomes from proximal aortic surgical repair.




Comments