Cardiogenic Shock’s Origins, Progression Tightly Tied to Mortality
Risk is lower when the etiology is HF versus MI, and both baseline and maximum shock severity matter, registry data show.

For patients with cardiogenic shock, the in-hospital mortality risk depends on the condition’s etiology—it’s substantially lower when it’s derived from acute decompensated heart failure (HF) than from myocardial infarction, registry data from the Cardiogenic Shock Working Group (CSWG) confirm.
Moreover, the condition’s severity at baseline and its maximum severity during the course of treatment also are intertwined with the risk that patients will die during hospitalization.
“Risk stratifying patients with cardiogenic shock remains a very important unmet need,” researcher Shashank S. Sinha, MD (Inova Heart and Vascular Institute, Falls Church, VA), told attendees at the Technology and Heart Failure Therapeutics (THT) 2022 meeting last week.
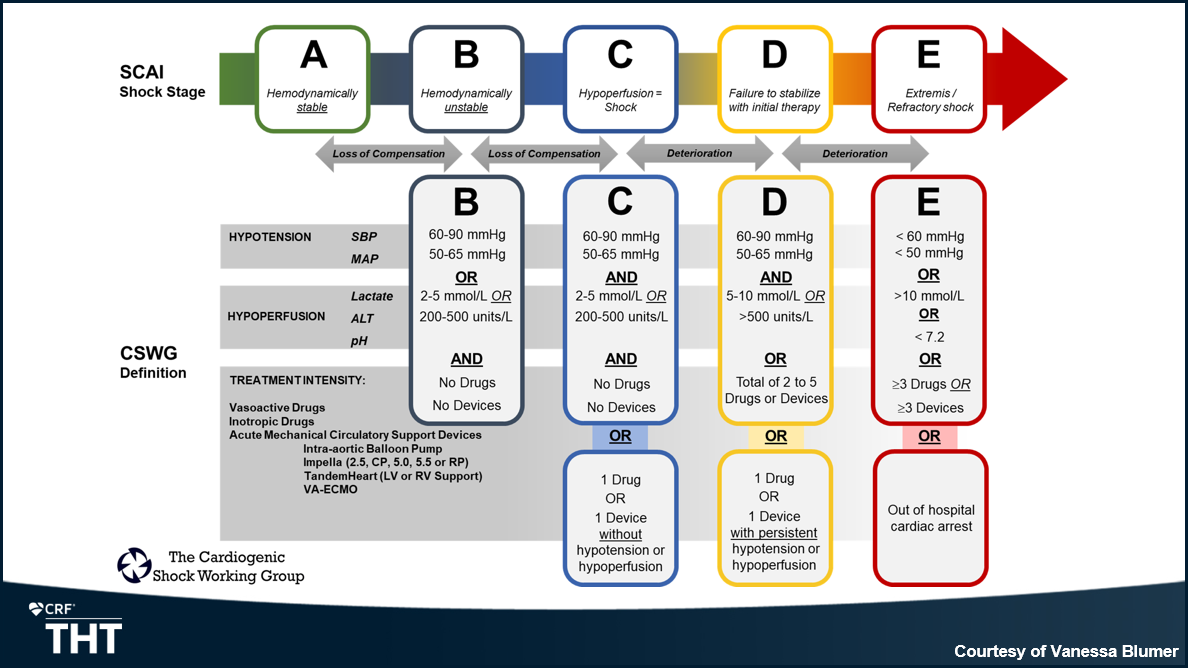
Cases were grouped using the Society for Cardiovascular Angiography and Interventions (SCAI) criteria, first released in 2019 and updated earlier this week: from stage A (At Risk) to stages B (Beginning), C (Classic), D (Deteriorating), and E (Extremis). While Sinha said the latest version of SCAI SHOCK continues to prioritize “simplicity and applicability,” he pointed out that it now more clearly conveys the fluidity of these scenarios.
In its revised classification pyramid, “what you see are shades of different colors bleeding into the escalating stage. . . . That was by design, to show that there are gradations of severity within each stage,” he explained. “More importantly, we’ve also learned that not all shock is created equal.” With acute MI complicated by cardiogenic shock (AMICS), “we have patients who initially present with hypotension, leading to hypoperfusion and then congestion as a late manifestation,” said Sinha, whereas HF patients “initially present with congestion.”
Srihari S. Naidu, MD (Westchester Medical Center, Valhalla, NY), who led the 2022 SCAI SHOCK effort, credited the CSWG for its input in developing the document. “We were in discussions with them throughout, and that’s part of the reason why it’s good to keep everybody abreast of where the field is going, so we can be very consistent.”
He said their analysis is a “next step,” in that explores the concepts illustrated in the SCAI document’s three-axis model for evaluation and prognostication, which in addition to shock severity highlights phenotype/etiology and risk modifiers. “You have to take the SCAI SHOCK stages and put them into context with the various types of variables that modify that risk,” Naidu explained.
“What they did in this study was . . . show that [AMICS] does indeed have higher mortality, no matter how you cut it, than heart failure-derived cardiogenic shock,” he noted. Additionally, the CSWG data on baseline and maximum severity confirm the idea “that wherever you are, the shock stage is predictive, and I think the higher it is and the longer that [it’s] at a high stage during the course of your stay, the more problematic it is from a survival standpoint.”
Nearly 3,500 Cases
To dig further into the relationship between severity, etiology, and outcome, Sinha and colleagues looked at 3,455 cases included in the CSWG registry between 2016 and 2021. HF patients made up 52% of the cohort and 32% presented with MI, while the rest had neither MI nor HF. SCAI SHOCK categories were retrospectively assigned, having been adjudicated by the study’s central committee.
Overall mortality was 35%, but the death rate was much higher with AMICS (42%) than with HF cardiogenic shock (25%).
“Broadly speaking, there was a gamut of short-term percutaneous, temporary mechanical support devices used throughout the registry, with intra-aortic balloon pump being the most common,” Sinha pointed out. Fully 42% of the HF patients were managed pharmacologically without a device, as compared with 15% of AMICS.
Treatment intensity—the number of drugs and/or devices used per patient—was linked to higher mortality risk in their data set, he said. The association was seen in both cohorts. For instance, with neither drug nor device therapy, the death rates were 14.3% for AMICS and 5.5% for HF cardiogenic shock. On the other end of the spectrum, with the use of at least four drugs and/or devices, death rates reached 70.6% for AMICS and 64.7% for HF cardiogenic shock.
With this knowledge, and the SCAI SHOCK system as a foundation, the CSWG developed a graphic that captures the “dynamic process” of the condition, Sinha said. It shows cutoffs for hypotension and hypoperfusion across stages A to E, which have been validated using their database, as well as treatment options.
Their registry data also revealed clear patterns in relation to shock severity and mortality when analyzed by baseline and maximum SCAI SHOCK stage. What the data indicate is that for stage C patients, “there does seem to be a treatment effect, perhaps initiation of drug or device does seem to have some benefit,” he observed. From both perspectives—baseline and maximum—stage C patients had the lowest mortality rate. At maximum stages B, C, D, and E, for instance, rates were approximately 15%, 5%, 20%, and 60%, respectively.
“What you see is that, instead of a stepwise progression, from B to C to D to E, we actually see a slight drop going from B to C,” Sinha noted to TCTMD. “We suspect—obviously this is postulation—that because they’re being treated with either a drug or a device, that there’s some improvement in that immediate mortality.”
A separate analysis stratified survival by acute MI and HF cardiogenic shock. “We do see, in fact, that there are key differences between these phenotypes,” Sinha said in his THT presentation. Mortality was substantially higher for AMICS versus HF at baseline of stages D and E as well as at maximum stages of C, D, and E. Moreover, AMICS mortality rose progressively along with maximum SCAI stage, whereas for HF, it was lowest at stage C.
Fine-tuning the Phenotypes
Naidu did point out one thing “that’s not congruent” between the SCAI SHOCK classification and the CSWG’s definitions: the role of hypoperfusion.
Their figure (above) lists either hypoperfusion or hypotension as criteria for stage B, and sets the lactate level at 2 to 5 mmol/L. In SCAI SHOCK, he noted, merely having a lactate level ≥ 2 mmol/L will automatically place a patient in stage C, irrespective of hypotension.
“That’s because we believe that hypoperfusion is dramatically worse and is therefore shock, because you have decreased perfusion that’s increasing anaerobic metabolism causing elevated lactate,” Naidu explained, referring to a 2021 paper linking hypoperfusion with mortality. With the less-stringent lactate cutoff, potentially “there’s going to be a significant number of patients that people ‘sit on’ in B and may not be as aggressive as they should be, because they think it’s stage B and not stage C,” he said. “So I’m a little worried about that.”
Navin Kapur, MD (Tufts University, Boston, MA), senior author of the new study and head of the CSWG, said that one challenge with SCAI SHOCK are words like “typically, maybe, and likely,” which leave room for interpretation. He served on the writing committee for both the 2019 and 2022 documents. “When I go around and talk to clinicians around the planet, basically they say: ‘We don’t know how to use SCAI stages, because it’s all opinion,’” he told TCTMD.
Thus, they wanted to come up with specific parameters and cutoffs derived from the CSWG data. “By putting in ‘or’ and ‘and,’ it makes it much more useful. Also, these are all parameters that every doctor . . . can acquire very quickly,” Kapur added. “Our attempt here is to try to create uniformity. We anticipate that this is going to adapt and evolve, but it’s the first time we’re starting to put granular, simple, accessible, and validated parameters into a scheme. . . . In fact, in the consensus document, they call for uniform definitions going forward. This is our proposal based on 3,500 patients, [with] 25 centers in the US, Europe, and Australia contributing to the data.”
From his perspective, the CSWG definition for stage B actually catches patients earlier in the spectrum of shock, in that it doesn’t require elevated lactate—no matter the cut point—at all. Hypotension alone is enough. “We wanted to get people recognizing shock earlier, so that you don’t push patients to C, classic shock, which is really probably too late,” Kapur explained. At 2 mmol/L, “even if your blood pressure is normal, your risk of dying is higher. And that’s why it shouldn’t be ignored.”
Kapur and Sinha, in a joint interview with TCTMD, asserted that SCAI SHOCK implies not only hypoperfusion but also hypotension are needed to reach stage C, thus setting the bar higher. Naidu said this isn’t the case: “In our model, you just have to have hypoperfusion—you don’t have to be hypotensive.”
Prognostication, Renal Function
All agreed that exploration of cardiogenic shock’s many manifestations will further the field. “We so desperately need more information in this area,” said session co-moderator JoAnn Lindenfeld, MD (Vanderbilt University Medical Center, Nashville, TN), following Sinha’s THT presentation.
She pointed out a key difference between the two etiologies, however: hospital stays are likely much shorter for AMICS patients, who tend to be treated rapidly, than for chronic HF patients, who “are often wandering around from floor to floor.”
Sinha replied that they are working on an analysis that looks at time to maximum SCAI SHOCK stage for both phenotypes.
Clyde W. Yancy, MD (Northwestern University Feinberg School of Medicine, Chicago, IL), also stressed that cardiogenic shock merits ongoing attention, “because we’ve continued to work in an ICU setting with a multiplicity of vasoactive drugs without any real guidance on whether that’s appropriate, or more importantly whether that’s hurting or helping.”
He suggested overlapping this CSWG framework with “well-established morbidity indices, so you can demonstrate to what extent this better discriminates who’s at risk compared with what we already know to use.” Another key detail worth exploring is renal function, Yancy added. “As a clinician at the bedside, when we see the hemodynamic shock and concomitant renal insufficiency that’s progressive, that seems to be the herald of [worse outcomes].”
Such analyses are forthcoming, said Sinha, with comparisons to risk scores including CardShock and IABP-SHOCK II. There also will be investigations into differences within the acute HF group based on whether cases are de novo or develop from chronic HF. He agreed that Yancy’s point about kidney function is “very valid.”
“There was robust discussion, as you might imagine, regarding renal function,” Sinha noted. The difficulty lies in how to define baseline creatinine, whether that’s a 90-day period, the week prior to hospital admission, or the day of hospitalization. “It became challenging except at the extremes, where perhaps looking at need for renal replacement therapy or significant doubling of their creatinine would be easier. That’s the reason why we chose to focus on the ALT [alanine aminotransferase] cutoff.”
Caitlin E. Cox is Executive Editor of TCTMD and Associate Director, Editorial Content at the Cardiovascular Research Foundation. She produces the…
Read Full BioSources
Sinha SS. SCAI stage across hospitalization and in-hospital mortality among cardiogenic shock patients. Presented at: THT 2022. New York, NY. February 3, 2022.
Disclosures
- The Cardiogenic Shock Working Group is funded by institutional grants from Abbott, Abiomed, Boston Scientific, Getinge, and LivaNova.
- Sinha and Naidu report no relevant conflicts of interest.
- Kapur reports consulting, speaking, and serving as a PI for research funded by Abiomed and Getinge.





Comments