CCM-D Delivers Safe, Effective Integrated Therapy in HFrEF
The Integra-D trial shows the device, which provides cardiac contractility modulation and defibrillation, also eases symptoms.
MINNEAPOLIS, MN—A novel implantable device that provides both cardiac contractility modulation as well as defibrillation (CCM-D) successfully and safely delivers integrated therapy, while also improving symptoms, for patients who have heart failure with reduced ejection fraction (HFrEF), according to new data from the Integra-D trial.
Nir Uriel, MD (NewYork-Presbyterian, Columbia University Irving Medical Center, New York, NY), who presented findings from the first 100 patients enrolled in Integra-D at the Heart Failure Society of America (HFSA) 2025 Annual Scientific Meeting, said the device successfully treated all patients undergoing defibrillation testing plus was able to perform ICD functions while delivering appropriate CCM therapy.
“There was no inappropriate CCM and ICD interaction despite a hybrid circuit,” he reported, adding that 91% of patients were compliant with charging their device for at least 60 minutes every 10 days. Notably, heart failure symptoms improved or remained stable in most patients.
“CCM-D provides an integrated therapy for heart failure patients, offering both symptomatic relief and sudden cardiac death prevention,” Uriel said. “As a clinician, we all know when we put an ICD, it’s a warranty. When we can put a CCM-D, it’s probably a warranty together with some heart failure symptom relief.”
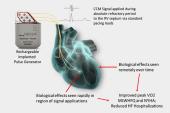
The Optimizer Integra CCM-D implantable pulse generator (Impulse Dynamics) is a next-generation device building off the Optimizer Smart/Smart Mini system, which was initially approved by the US Food and Drug Administration in 2019 to improve functional status and symptoms in patients with HFrEF who are symptomatic despite optimal medical therapy. CCM is designed for patients who are not indicated for cardiac resynchronization therapy (CRT), have an LVEF ranging from 25% to 45%, and are in normal sinus rhythm.
The advantage with CCM-D is that patients who are indicated for both CCM and ICD therapy can be implanted with a single device instead of two.
Integra-D Findings
The analysis included 101 patients (mean age 62.5 years; 28.7% female) who were indicated for de novo ICD without CRT, with stage C/D heart failure on guideline-directed medical therapy, and with an LVEF of no more than 40%. Mean LVEF at baseline was 30%, and 93% were considered to have class II or III NYHA symptoms. Half of patients were taking at least triple therapy for heart failure at baseline, and 35.6% were taking quadruple therapy including an SGLT-2 inhibitor.
The Optimizer Integra device was successfully implanted and induced in 100 patients, with defibrillation testing resulting in complete success. Complications at 30 days included one patient with a lead dislodgement and one patient with an infection that was treated with antibiotics. One patient also reported worsening heart failure, and two patients had events classified as “other,” including one non-HF related hypotensive event and one instance of pericarditis that improved on colchicine.
CCM assessment resulted in no inappropriate treatment during ventricular arrhythmias and no CCM leading to inappropriate ICD oversensing. Approximately nine in 10 patients received the recommended CCM dose of at least 70% by 30 days.
At 6 months, 88% of patients reported no change in NYHA symptom class and 7% had improved by two classes. Among the 11 patients who saw worsening symptoms, six were noncompliant with charging their devices or had adverse events close to the time of assessment.
Compared with an outside control group of patients receiving CRT-D therapy in COMPANION, “nearly identical” NYHA class improvement was seen with CCM-D in this study among patients labeled as NYHA class II/III at baseline, Uriel reported.
Mechanistic Questions
While CCM therapy has been available for several years, Carlos Santos-Gallego, MD, PhD (Icahn School of Medicine at Mount Sinai, New York, NY), who was not involved in the study, told TCTMD that it hasn’t been as popular with heart failure physicians as proponents might hope despite “massive” quality-of-life gains seen in prior research. “It’s a system that seems to work, but we do not really understand why it works,” he said. “Hence, we are not using it too much.”
He likened the phenomenon to the pathway that SGLT2 inhibitors took to find success in the field. “They were found to be initially useful for heart failure in 2016, but then for 2 or 3 years everybody was like, ‘Oh, we are not using them in heart failure. God knows how they work,’” Santos-Gallego said. “Once that we knew how they were working and there were examples of confirmatory trials, then they started to be used.”
Daniel Burkhoff, MD, PhD (Cardiovascular Research Foundation, New York, NY), presented a related study during the meeting, that one showing patients who got CCM had significantly better event-free survival at 18 months compared with a matched group of control patients receiving CRT (P = 0.002). He agreed that CCM has faced challenges with adoption.
“A lot of physicians still don’t know about it,” he told TCTMD, adding that many are also looking for more evidence on how it might reduce heart failure hospitalizations as well as mortality. “The data are pretty strong about exercise tolerance and quality of life. . . . Hopefully, payers are starting to recognize that a big part of what devices do is improve quality of life in heart failure.”
To fully understand what a device or even a drug does to improve outcomes is not so simple, Burkhoff said. “The fundamental concept that started this was that these electrical signals improve calcium cycling and enhance the strength of the heart,” he explained. “But over the years in many labs around the world there have been other mechanisms that have been shown to contribute both in the short and the long term. And that’s very typical of all therapies, including CRT.”
Burkhoff continued: “It really goes to show, although we like to say we know the mechanism, a lot of times we kid ourselves.”
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Uriel N. Effects of a novel cardiac contractility modulation-defibrillator (CCM-D) on NYHA functional class in ICD-indicated patients: post-implant results from the Integra-D trial. Presented at: HFSA 2025. September 28, 2025. Minneapolis, MN.
Burkhoff D. Cardiac contractility modulation reduces mortality and heart failure hospitalizations: a matched comparison of patients receiving and not receiving CCM derived from a real-world dataset. Presented at: HFSA 2025. September 28, 2025. Minneapolis, MN.
Disclosures
- Uriel reports conflicts of interest with Abbott Laboratories, Abiomed, LiveMetric, Revamp, and Leviticus.
- Burkhoff reports serving as a consultant to Impulse Dynamics.
- Santos-Gallego reports no relevant conflicts of interest.




Comments