FDA Approves the Optimizer Smart Implantable Pulse Generator for Heart Failure
The device delivers cardiac contractility modulation and is indicated for use in chronic heart failure patients who are not eligible for CRT.
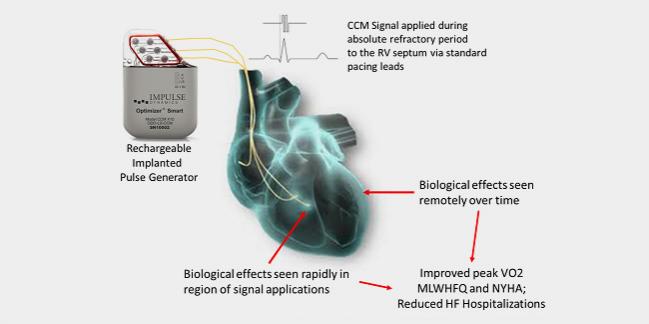
The Optimizer Smart implantable pulse generator, which delivers cardiac contractility modulation (CCM) therapy, is now approved by the US Food and Drug Administration for the treatment of chronic, moderate-to-severe heart failure in patients who are not suited for treatment with other devices such as cardiac resynchronization therapy (CRT). Device Impulse Dynamics announced the approval yesterday.
Optimizer Smart was given “breakthrough device” designation by the FDA in 2015, and according to its manufacturer was the first of such to be presented before the agency’s Circulatory System Devices Panel, which in December voted 12-0 with one abstention in favor of the benefit-to-risk ratio. The panel also voted 12-1 and 11-2 on questions of safety and efficacy, respectively.
Designed to deliver electrical pulses to the right ventricular septum of the heart during the myocardial absolute refractory period, the Optimizer Smart device received CE Mark approval in October 2016, and earlier versions of the device have been implanted in more than 3,500 patients worldwide over the past decade.
The primary evidence reviewed by the panel and the FDA came from the FIX-HF-5C trial, designed to confirm subgroup findings of the earlier FIX-HF-5 trial, which did not meet its primary endpoint for ventilatory anaerobic threshold but did show a benefit for CCM over optimal medical therapy with regard to peak oxygen consumption (pVO2) and quality-of-life measures at 6 months. The newer study showed improvements in exercise tolerance as measured by pVO2 (primary endpoint), quality of life on the Minnesota Living With Heart Failure Questionnaire score and 6-minute hall walk test, and NYHA functional status at 24 weeks with CCM compared with optimal medical therapy. CCM was also shown reduce the primary safety composite endpoint of cardiovascular death and heart failure hospitalization.
“With the FDA’s approval of the Optimizer System for the delivery of CCM, we finally have available in the US an effective device-based therapy for advanced heart failure patients with mildly to moderately reduced left ventricular ejection fractions who are not eligible for CRT,” said William Abraham, MD (The Ohio State University Wexner Medical Center, Columbus), in a press release. “The Optimizer System, along with guideline-directed medical therapies, can improve the lives of many heart failure patients in the US who previously did not have access to this therapy. As such, it represents a real game-changer for these patients.”
“FDA approval is the culmination of many years of clinical development for this disruptive technology, addressing a significant unmet need in today’s heart failure treatment paradigm,” Impulse Dynamics’ medical advisor Daniel Burkhoff, MD, PhD (Cardiovascular Research Foundation, New York, NY), noted in the same press release. “We continue to develop the technology with ongoing clinical trials designed to evaluate CCM therapy in additional heart failure populations.”
The manufacturer says that it plans to launch the device in the United States later this year.
Note: Burkhoff is a faculty member of the Cardiovascular Research Foundation, the publisher of TCTMD.
Photo Credit: Abraham WT. Cardiac Contractility Modulation for HFrEF and HFmrEF. Presented at: TCT 2018. September 22, 2018. San Diego, CA.
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Impulse Dynamics. Impulse Dynamics receives FDA approval for breakthrough Optimizer Smart System delivering CCM therapy for treatment of heart failure. Published on: March 21, 2019. Accessed on: March 21, 2019.


Comments