CTO PCI Improves Total Myocardial Viability, PET Imaging Suggests
Recanalizing a CTO seems to benefit both the target area and remote myocardium, but outcomes data are still needed.
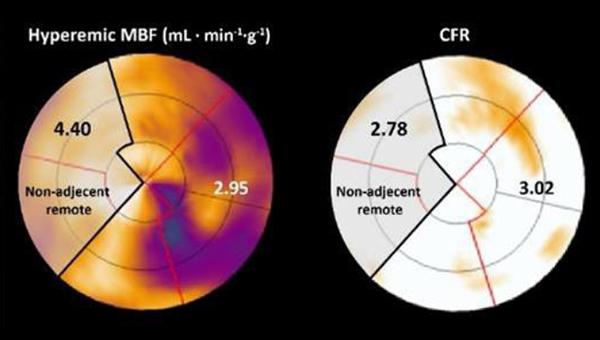
Photo Credit: Adapted from de Winter RW, et al. EuroIntervention. 2021;Epub ahead of print. Used with permission.
Remote myocardial perfusion increases after revascularization of a chronic total occlusion (CTO) lesion, with the biggest gains seen in patients who have the greatest improvements in various measures of blood flow, new research shows. This suggests that CTO PCI “may have a favorable physiologic impact” beyond the targeted myocardium, the authors say.
While the study does nothing to answer the long-held question of whether CTO PCI affects hard patient outcomes, it does “add a small piece in the physiological puzzle of coronary hemodynamics in the presence of a CTO,” lead author Ruben de Winter, MD (Amsterdam University Medical Center, the Netherlands), told TCTMD.
“It's important to realize that there is a complex interaction between the CTO vessel and the other coronary arteries through the collateral channels that are present, and that the complete situation changes after a CTO is revascularized,” he explained. “What we have seen is that it may not only improve the absolute perfusion in the CTO vessel itself, but it may also be beneficial for the other coronary arteries, so the total myocardium, after CTO is revascularized.”
Commenting on the study for TCTMD, Nieves Gonzalo, MD, PhD (Hospital Clinico San Carlos, Madrid, Spain), said in an email that the likely mechanism behind this phenomenon comes from relief of collateral arteries that had developed to supply blood to the CTO territory, and hence a lowering of the overall workload placed on the remote myocardium. “Prior studies have shown the complex hemodynamic interplay between the myocardium subtended by the donor artery and the CTO territory,” she added.
Additionally, Rushi Parikh, MD (David Geffen School of Medicine at UCLA, Los Angeles, CA), told TCTMD that the study “elegantly complements” existing interventional data in this space, although those studies have all been small and observational, as well, and not “well appreciated.”
What the study by de Winter and colleagues “hammers home is that the benefit of CTO PCI isn’t just in that territory, but also in the donor-vessel territory where you have this relative ischemia, is the way I think about it,” he continued. “Let’s say the LAD is supplying collaterals to the right. [After CTO PCI], you don't need the LAD to also provide blood flow to the right coronary territory. Now it can just subtend the LAD territory, and so what this study showed is that the coronary flow reserve and the hyperemic myocardial blood flow on PET increases in both territories.”
Increases in Remote Myocardial Perfusion
For the study, published online last week in EuroIntervention, de Winter and colleagues performed serial PET perfusion imaging at baseline and at 3-month follow-up in 164 patients who underwent CTO PCI. They found a positive correlation in resting myocardial blood flow (MBF), hyperemic myocardial blood flow (hMBF), and coronary flow reserve (CFR) between the CTO and remote myocardium before and after CTO PCI (P < 0.01 for all).
Additionally, there were absolute increases in hMBF (2.29 to 2.48 mL·min–1·g–1) and CFR (2.48 to 2.74) in the remote myocardium after CTO PCI. These improvements were greatest in patients with higher increases in hMBF (β 0.58; 95% CI 0.48-0.67) and CFR (β 0.54; 95% CI 0.44-0.64; P < 0.01 for all) in the CTO territory, regardless of clinical, angiographic, and procedural features.
Interestingly, there were no differences in changes in myocardial perfusion indices between patients with and without prior MI.
Before this study, de Winter said he thought there was a connection between remote coronary arteries and the CTO vessel in that revascularization would alter the entire picture. “What we have seen is that it not only increases the perfusion in the CTO vessel, but it also maybe increases the absolute myocardial blood flow in the myocardium that was not initially the target vessel,” de Winter explained. It’s a “really complex” process, he continued, “but we hypothesized that after the collaterals regress because the myocardial mass is diminished and the coronary donor arteries don't have the supply the CTO myocardium anymore, it may actually improve the perfusion pressure in the remote vessels.”
Small studies using invasive physiology and MRI “have failed to demonstrate perfusion increases in remote myocardium after CTO PCI,” Gonzalo said, suggesting that “discrepancies with the current study can be explained by their limited sample size and the different diagnostic tools used.”
Also, previous studies have suggested that revascularizing a CTO can increase fractional flow reserve “in the donor artery by reducing the collateral flow,” she continued. “This makes the increase in remote myocardial perfusion induced by collateral regression after CTO PCI a plausible hypothesis.”
Xiantao Song, MD, PhD (Beijing Anzhen Hospital, China), also commenting on the study for TCTMD, said in an email that the correlation between perfusion indices in remote and CTO myocardium “might be largely attributable to the extent of microvascular disease,” a topic previously hinted at but not directly explored in a 2019 study. As such, Song said the results of this latest analysis by de Winter et al suggests that CTO PCI is “capable” of preventing future heart failure and myocardial damage that could lead to arrhythmias, although “myocardial viability should be determined before CTO PCI.”
Effects on Patient Selection
As for how these data might affect future patient selection of CTO PCI, de Winter said it is already known that lower CTO perfusion at baseline leads to a larger increase in absolute MBF, though this study confirms that if “you select the patients that are likely to have the largest increase in perfusion in the CTO vessel after revascularization, then it might also result in the largest increase in remote myocardial perfusion.”
Gonzalo agreed that “the study suggests that work overload in the donor territory might be relieved by CTO PCI” and said its findings could help with patient selection, although the specifics remain murky. “You could consider that increasing the perfusion in the donor territory would be more beneficial if it is a large territory,” she said. “On the other hand, if there is a large donor territory with different donor arteries the ‘work overload’ is more distributed and therefore the impact would be lower.”
While some people might think that well-developed collaterals are “good enough” in a patient with a CTO, “there's plenty of data to show that it's a good stopgap, but it's not good enough. You still are ischemic in that territory,” Parikh said, adding that CTO PCI will increase CFR and hMBF even in patients with good collateral flow. “But then what I think people don't intuitively think about is with the collaterals regressed in a way that’s now improving blood flow to the territory where the collaterals were coming from in the first place—because now they don't have to shunt blood to this territory so you’re getting incremental blood flow there—the question is: are there subgroups of patients where this may be more beneficial?”
The study found no difference in patients with and without prior MI, but he said that isn’t surprising because of infarct damage in those territories. Parikh would like to see a closer look at how reduced EF might affect post-CTO PCI blood flow, especially in a larger study. “If you have EF of 35-40% and you do CTO PCI, does that reduction in relative ischemia in donor territory actually provide a greater incremental benefit [to] LVEF than normal, in the sense that you're improving LVEF so maybe CTO PCI is more warranted/beneficial in those patients?” he asked.
The next big step should be determining whether increasing myocardial perfusion in the remote myocardium brings about clinical benefits, Gonzalo agreed.
In addition to outcomes data, de Winter said he would also like to see more work combining “all the physiological measures” following CTO PCI. “In our study, the limitation is that we do not know what the fractional flow reserve did in the remote coronary artery after CTO PCI, so we couldn't relate those changes to the increase in absolute myocardial perfusion,” he said, adding that the ongoing IMPACT CTO 2 trial should add to the knowledge base.
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
de Winter RW, Schumacher SP, van Diemen PA, et al. Impact of percutaneous coronary intervention of chronic total occlusions on absolute perfusion in remote myocardium. EuroIntervention. 2021;Epub ahead of print.
Disclosures
- De Winter, Gonzalo, and Parikh report no relevant conflicts of interest.





Comments