Digitoxin Cuts Risk in Well-Treated HFrEF Patients: DIGIT-HF
(UPDATED) The effect size was modest, but the glycoside improved clinical outcomes when added to GDMT.
MADRID, Spain—Adding digitoxin, a cardiac glycoside, to contemporary guideline-directed medical therapy (GDMT) improves clinical outcomes in patients who have heart failure with reduced ejection fraction (HFrEF), according to results from the DIGIT-HF trial.
Treatment with digitoxin cut the absolute risk of all-cause mortality and hospitalization for worsening HF by 4.6% compared with placebo over a median follow-up of 36 months (HR 0.82; 95% CI 0.69-0.98).
Lead investigator Udo Bavendiek, MD (Hannover Medical School, Germany), who presented the results today during the first Hot Line session at the European Society of Cardiology Congress 2025, said the overall benefit of digitoxin was consistent in patients who were taking an angiotensin receptor-neprilysin inhibitor (ARNI) or sodium-glucose cotransporter 2 (SGLT2) inhibitor at baseline, as well as in those taking triple or quadruple combinations recommended by clinical guidelines.
To TCTMD, Bavendiek acknowledged the difficulties of getting patients who are already taking multiple medications just for HF, let alone other conditions, to add yet another drug. “Of course, it’s a challenge to get all patients on the drugs which improve outcomes, especially mortality, but it was possible in our trial population,” he said.
The researchers hope to delve into the data in greater detail to potentially identify those who stand to benefit most from digitoxin. In a subgroup analysis, for example, patients without an implantable cardioverter-defibrillator (ICD) at baseline appeared to derive a larger reduction in the risk of all-cause mortality or admission for worsening HF than those without an ICD. Bavendiek said this finding surprised them, and they plan to look into ICD protocols to better understand the result.
“If you have to add another medication, I think you have to really specify which patients would likely profit, but overall, I think, it’s important that we have a clearly positive signal in the data,” he said.
Michelle Kittleson, MD, PhD (Cedars-Sinai Medical Center, Los Angeles, CA), who wasn’t involved in the study, said DIGIT-HF was an important clinical trial to perform as it’s been nearly three decades since the DIG trial testing digoxin in patients with chronic HF was published.
For Kittleson, DIGIT-HF is a welcome addition, but she is not convinced it should change clinical practice given the relatively small absolute effect size. Additionally, there were almost twice as many serious adverse events with digitoxin when compared with placebo.
“Patients are already at risk for polypharmacy, and quadruple therapy remains challenging to implement in HFrEF,” she told TCTMD. “Until there is a better understanding of the serious adverse events and causes of death, I would spend my energy achieving target doses of the ARNI, evidence-based beta-blocker, MRA [mineralocorticoid receptor antagonist] therapy, and SGLT2 inhibition in HFrEF prior to consideration of cardiac glycoside therapy.”
Carlos Santos-Gallego, MD, PhD (Icahn School of Medicine at Mount Sinai, New York, NY), another HF expert, said that DIGIT-HF is clearly a win for digitoxin in this patient population. The 18% relative reduction in risk is significant but not as “clinically relevant” as the 35% reduction in HF hospitalizations with SGLT2 inhibitors.
“Therefore, the four pillars of treatment—ARNI, beta-blockers, MRA, and SGLT2 inhibitors—will continue as clear first-line therapy, but digitoxin seems to be a great option for those patients who remain symptomatic despite being on the four pillars,” he explained.
As the discussant following the Hot Line presentation, Theresa McDonagh, MD (King’s College London, England), said that registry studies suggest that just 15% of HFrEF patients are on digoxin. Over the years, the recommendations to use digoxin have been downgraded, but she suspects DIGIT-HF will warrant a rethink, with a 2a recommendation possible in future guidelines.
“I think this will also change clinical use for both digoxin and digitoxin,” she said. “We’ll see more use of cardiac glycosides in advanced heart failure [patients] in sinus rhythm and atrial fibrillation.” Digitoxin, she added, may be used more given its more favorable safety profile.
Optimized on GDMT
Digitoxin is pharmacodynamically similar to digoxin, but the drug can be eliminated by the liver when renal function is impaired. Digoxin, on the other hand, is predominantly excreted by the kidneys. Digitoxin is considered a safer cardiac glycoside than digoxin, with serum concentrations remaining stable without dose adjustments.
In the DIG trial, digoxin failed to reduce the risk of all-cause mortality, but treatment cut the risk of hospitalization for worsening HF. The effect of digoxin on HF hospitalization has been backed up from other retrospective studies and meta-analyses. In the 2022 US guidelines for the management of HF, digoxin can be considered for HFrEF patients who remain symptomatic despite GDMT, or who are unable to tolerate GDMT (class 2b recommendation, level of evidence B).
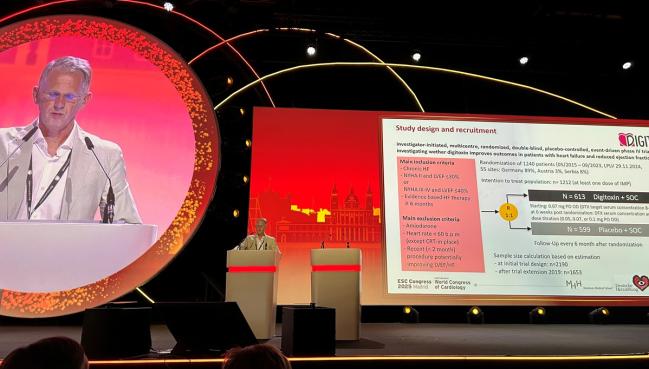
DIGIT-HF, which was published simultaneously in the New England Journal of Medicine, took 10 years to complete, with the trial stopping short of planned enrollment after funding evaporated. Investigators ultimately randomized 1,240 patients (mean age 66 years; 20.4% women) with chronic HF, left ventricular ejection fraction less than 40%, and NYHA functional class III symptoms (or those with LVEF less than 30% and NYHA functional class II symptoms) to digitoxin or placebo.
In the end, the intention-to-treat analysis included 1,212 patients: 25 patients who underwent randomization were excluded as they never received a dose of digitoxin or placebo and three patients were excluded after the clinical site closed due to noncompliance with trial standards.
Overall, 93% were treated with a beta-blocker and a renin-angiotensin-system inhibitor, including nearly 40% who were taking an ARNI. More than 76% of patients were also taking an MRA, and nearly 20% were taking a SGLT2 inhibitor. With devices, 64.3% were treated with an ICD and 25.2% with cardiac resynchronization therapy.
The primary outcome—a composite of all-cause mortality or hospital admission for worsening HF—occurred in 39.5% of the 613 patients randomized to digitoxin and 44.1% of the 599 randomized to placebo, a difference that was statistically significant (P = 0.03). There was a favorable effect of digitoxin on the individual components of the primary outcome, but the differences were not statistically significant.
The benefit of digitoxin appeared similar in both men and women, an important finding given the sex-based differences in outcomes in the DIG study. There, digoxin was associated with an increased risk of death from any cause among women, but not men.
Serious adverse events occurred in 4.7% of those treated with digitoxin compared with 2.8% of those randomized to placebo. Cardiac disorders occurred in 3.4% of those who received digitoxin, with 17 events due to ventricular fibrillation (1.6%) or tachycardia (1.1%).
Digitoxin Serum Levels
In a post-hoc analysis of the DIG trial, investigators showed that higher serum digoxin concentrations were associated with increased mortality, whereas low serum concentrations were not. In DIGIT-HF, those treated with digitoxin received a 0.07-mg dose once daily and adjustments were made in a blinded manner to keep serum concentrations in a predefined target range of 8 to 18 ng/mL.
“We know from the DIG trial data that serum concentrations at the lower range are important, so our target range was adopted based on the digoxin data,” said Bavendiek. “One reason why we used digitoxin was that it’s really simple to dose. We do not have to repeatedly [measure] serum concentrations, because it’s not accumulating in a patient with kidney disease because [there’s] compensatory excretion by enterohepatic circulation.”
DIGIT-HF, notably, enrolled more severely ill patients than in other trials, such as PARADIGM-HF, DAPA-HF, and EMPEROR-Reduced, said Santos-Gallego. While LVEF and the presence of cardiovascular risk factors were comparable across trials, DIGIT-HF enrolled more patients with NYHA functional class III and IV. While heart rates were similar, ivabradine use was also higher in DIGIT-HF.
Regarding the lack of a significant reduction in all-cause mortality or hospitalization for worsening HF when assessed alone, Santos-Gallego noted that DIGIT-HF enrolled just 55% of the originally designed sample size. Additionally, the number of events was much smaller than anticipated. Had the trial enrolled the planned 2,190 patients and accrued the expected number of events, it most likely would have been positive, he said.
One of the more curious aspects of the trial was that event curves separated early, remained apart in the midterm, and then came back together during follow-up, said Santos-Gallego. This might be explained by a low number of clinical events over the long term. With a smaller-than-expected trial, “this means only a very reduced number of patients reach the 6 to 8 years of follow-up,” he said. “Hence, the number of events is extremely low and the curves tend to converge.”
Also, ARNIs were introduced into practice in 2015 and SGLT2 inhibitors in 2020, which likely means that more patients were on optimal GDMT in the later years of follow-up, said Santos-Gallego. This would make it harder to find differences in outcomes between treatment groups.
More Data to Come
Kittleson, part of the writing committee that drafted the HF guidelines, said that HF therapy has changed significantly since the 1997 DIG study, making DIGIT-IT an important trial to conduct.
“The authors should be congratulated on performing a much-needed study of a generic medication,” Kittleson said. “It’s refreshing because it’s aimed at addressing an evidence gap rather than solely FDA approval of a novel medication, as economics often prioritize the latter goal over the former.”
Santos-Gallego noted that the Dutch DECISION trial is ongoing, and though that study is evaluating digoxin in symptomatic HFrEF patients, the data will provide a complementary view of the efficacy of digitalis. He noted, however, that digoxin is sparingly used in clinical practice because of its poor safety, and that digitoxin is no longer available in the US. After DIGIT-HF, he expects that to change.
Michael O’Riordan is the Managing Editor for TCTMD. He completed his undergraduate degrees at Queen’s University in Kingston, ON, and…
Read Full BioSources
Bavendiek U, Groβhenning A, Schwab J, et al. Digitoxin in patients with heart failure with reduced ejection fraction. N Engl J Med. 2025;Epub ahead of print.
Disclosures
- Bavendiek report grant support from Alnylam Pharmaceuticals, the German Heart Foundation, and the German Federal Ministry of Education and Research. He reports having speaking engagements and/or serving on the advisory board and/or travel support from Alnylam, Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Eli Lilly, Novartis, and Pfizer.
- Kittleson reports no relevant conflicts of interest.




Comments