EXTRACT-PE: Positive Results With Aspiration Thrombectomy for Submassive PE
RV function improved after treatment with the Indigo system, with low rates of major bleeding and adverse events.
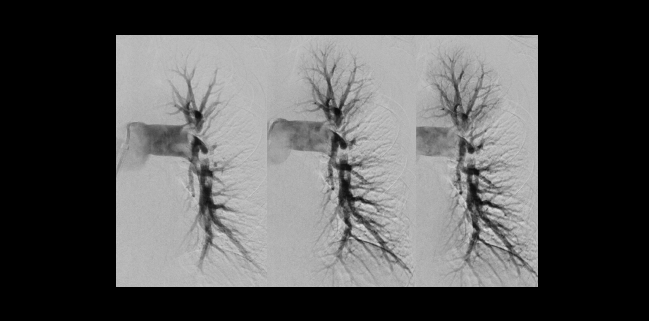
Photo Credit: Michael Rosenberg, MD (University of Minnesota, Minneapolis)
Among patients with submassive pulmonary embolism (PE), right ventricular function can improve within 48 hours of undergoing thrombectomy with the Indigo aspiration system (Penumbra), final results of the single-arm EXTRACT-PE trial show.
The RV/LV diameter ratio fell by 27.3% after the procedure. Three major adverse events occurred within 48 hours in two patients (1.7%)—both had major bleeds and one ultimately died, researchers led by Akhilesh Sista, MD (NYU Grossman School of Medicine, New York, NY), report. Intraprocedural thrombolytic therapy was avoided in all but two patients.
The findings, published online January 13, 2021, ahead of print in JACC: Cardiovascular Interventions, “absolutely” place the Indigo system in the operator’s toolbox for the treatment of acute PE, Sista told TCTMD. “It’s as far along as any aspiration device out there, at least based on the evidence.”
He noted that a lot more research is needed to sort out the best treatment approaches for patients with acute PE, particularly when it’s submassive, and how big of a role devices will fill.
“We are going to see an exponential growth of innovative devices in PE, and with COVID, with obesity, venous thromboembolism is going to become more prevalent,” Sista said. “The important thing for us to do as physicians and scientists is to ensure that we know that removing thrombus in the first place is a good thing for whatever population, and so we need fundamental, rigorous randomized trials of thrombus removal [done] the best way we know how to get to the point that these devices can be anchored to something. Because the danger right now is that all of these devices come out without that clear trial that tells us that it is the right thing to do, especially in submassive PE.”
EXTRACT-PE
There is uncertainty about the best approach for treating submassive PE, which is characterized by normal blood pressure and impaired RV function. A 2014 meta-analysis showed that systemic thrombolytic therapy was associated with a lower risk of all-cause mortality compared with anticoagulation alone, but carried a higher risk of major bleeding.
One solution to counter that bleeding risk is to use catheters—with or without ultrasound assistance—to deliver medications directly to the clot. The SEATTLE II study showed that ultrasound-assisted, catheter-directed thrombolysis using the EkoSonic system (Boston Scientific) was associated with a reduction in RV/LV ratio. Yet it had a 10% major bleeding rate within 72 hours.
Another option is percutaneous aspiration thrombectomy with or without the use of thrombolytics. Preliminary data were promising for use of the Indigo system, which received US Food and Drug Administration 510(k) clearance for pulmonary embolism in December 2019 based on initial results of EXTRACT-PE investigational device exemption trial.
Sista et al report on the full results of that study, which was conducted at 22 US sites. EXTRACT-PE enrolled 119 patients with signs and symptoms of acute PE for no more than 14 days, angiographic evidence of PE, a systolic BP of 90 mm Hg or higher, and an RV/LV diameter ratio greater than 0.9. All but one of the patients had submassive PE at the time of enrollment (one patient had massive PE). Mean patient age was 59.8, and 44.5% were women.
The thrombectomy procedure was completed in all but one patient—in that case, the procedure was aborted because of tortuous vessel anatomy. Only two patients (1.7%) received intraprocedural thrombolytic therapy.
Overall, the median time from device insertion to removal was 37 minutes. That’s “pretty low,” Sista said, “which is good in terms of PE because the longer you have a PE patient on the table, you’re increasing your odds of having that patient decompensate in a non-ICU environment potentially.”
The primary efficacy endpoint was the change in RV/LV ratio from baseline to 48 hours after the procedure. There was a median reduction 0.43 units, from 1.47 to 1.04 (P < 0.0001), a magnitude comparable to that seen in SEATTLE II and the FLARE trial, which evaluated the Flowtriever aspiration system (Inari Medical).
For safety, the primary endpoint was a composite of major adverse events within 48 hours—that included device-related death, major bleeding, and device-related serious adverse events (clinical deterioration, pulmonary vascular injury, and cardiac injury). Two patients had three major adverse events. One patient had a postprocedural access-site bleed in the groin and was stabilized with transfusion of two units of packed red blood cells. The other developed hemoptysis during the procedure stemming from a distal vessel perforation and then developed a postprocedural access-site bleed that required one unit of packed red blood cells. Several hours later, the patient developed sustained ventricular tachycardia, which eventually resulted in death.
Sista said he was encouraged by the device’s overall safety and its low rate of adverse events, but added the most serious event “is not something to ignore. And it speaks to the delicacy of the pulmonary arterial tree and that overexuberant use of catheters, wires, whatever components the device comes with comes with risk, and that especially in anticoagulated patients you can have very bad pulmonary complications.”
No patients had cardiac injury, and rates of pulmonary vascular injury, clinical deterioration, and major bleeding within 48 hours were all low at 1.7%. During 30 days of follow-up, the all-cause mortality rate was 2.5%, with no cases of symptomatic PE recurrence of intracranial bleeding.
Evidence Vacuum
Commenting for TCTMD, Kush Desai, MD (Northwestern University Feinberg School of Medicine, Chicago, IL), who wrote an accompanying editorial, said EXTRACT-PE is an important study because it provides some independent validation of FLARE, the only other prospective trial of mechanical thrombectomy in acute PE.
But there are a couple of important caveats, he said. For one, “nobody’s ever proven that RV/LV ratio is the right thing to look at to show that we’re actually making these patients better,” he said. “We need a much more rigorous, clinically derived endpoint, instead of showing that we’re reducing RV/LV ratio, which is really designed as a prognostic marker, not as an endpoint in and of itself.”
Along with FLARE, it shows you that, in general, in experienced hands, mechanical thrombectomy for pulmonary embolism is safe to do and that—using a surrogate endpoint—it shows some benefit. Kush Desai
Secondly, Desai said, a certain degree of variability is inherent to the performance of mechanical thrombectomy, making it hard to standardize practice across sites and provide evidence to guide operators. “That guidance isn’t there, and I think that’s a critical missing link,” he stressed.
Thus, Desai said, and Sista agreed, there is a need for a randomized trial to evaluate whether advanced interventions like aspiration thrombectomy and catheter-directed thrombolysis improve patient outcomes when compared with standard-of-care anticoagulation.
In the meantime, the decision to use one of these advanced techniques comes down to local experience and level of comfort and, perhaps most importantly, whether there is a multidisciplinary PE response team (PERT) available to help select appropriate patients, Desai said. “In the end I believe that that leads to—at least based on our current knowledge—the best decision being made.”
It’s tough to say how widespread use of these advanced modalities should be in the absence of additional evidence, he said. “I think that we are helping people. I just don’t think we have a good handle on how we’re helping people, how well we’re helping people, and the optimal patient selection. So in that vacuum, I think that we proceed with caution. [I think] that if you have this multidisciplinary type of setup with a PERT team, by and large you’re going to end up selecting patients—provided everybody’s honest—that are going to do well with these therapies, or at least not have a really adverse outcome with these therapies.”
For Sista, use of these devices for submassive PE “really depends on what you’re comfortable with, the local success you’ve had, local politics, what the multidisciplinary PE group at your institution believes in, and then what you consider to be strong enough evidence.”
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Sista AK, Horowitz JM, Tapson VF, et al. Indigo aspiration system for treatment of acute pulmonary embolism (PE) – results of the EXTRACT-PE trial. J Am Coll Cardiol Intv. 2021;Epub ahead of print.
Desai KR. Mechanical thrombectomy in pulmonary embolism: ready for prime time? J Am Coll Cardiol Intv. 2021;Epub ahead of print.
Disclosures
- The trial was funded by Penumbra.
- Sista reports unrestricted research support from Penumbra administered through his institution.
- Desai reports no relevant conflicts of interest, but reports relationships: serving on the speakers’ bureau/consulting for Cook Medical, Becton Dickinson, and Boston Scientific and consulting for W.L. Gore, Medtronic, Philips, Cardinal Health, and Tactile Medical.


Comments