FDA Approves Amplatzer Amulet LAA Occluder
“As with any cardiovascular procedure, it really is important to the landscape to have options,” Jonathan Hsu says.
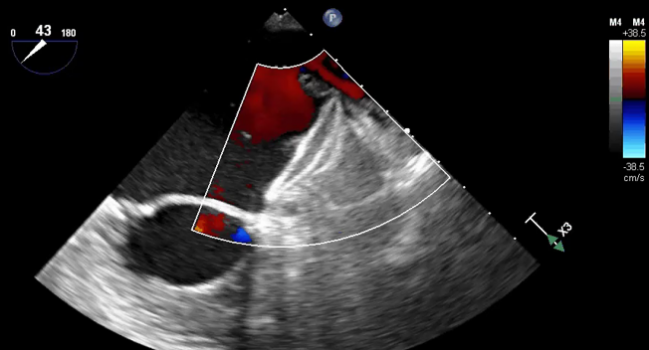
Photo Credit: Hermiller J. AMULET: device characteristics, procedural technique, clinical outcomes, and ongoing studies. Presented at: TCT 2019.
The US Food and Drug Administration has approved the Amplatzer Amulet left atrial appendage (LAA) occluder, bringing US physicians an additional option for device-based stroke prevention in patients with atrial fibrillation (AF), device maker Abbott announced Monday.
The Amulet—indicated for patients who are suitable for short-term anticoagulation but have a reason to seek an alternative to long-term treatment—enters a US market that has been the domain of the Watchman LAA closure device (Boston Scientific) since the first-generation product was approved in 2015; FDA approval of the next-generation Watchman FLX came last year.
Abbott said Amulet’s approval was based on the results of the Amulet IDE trial, a noninferiority trial comparing the occluder with Watchman devices, although the results will not be known until they’re presented at the virtual European Society of Cardiology Congress 2021 in a few weeks.
“As with any cardiovascular procedure, it really is important to the landscape to have options,” Jonathan Hsu, MD (University of California, San Diego), commented to TCTMD. “Having a different option in regards to left atrial appendage occlusion improves our chances of improving the therapy in patients with atrial fibrillation. . . . Future studies will inform us about who may benefit from one specific device versus another.”
What’s Different From Watchman
There are indeed some differences between the occluders, as the Watchman consists of a single component to block the LAA and the Amulet has both a disc designed to seal off the opening of the appendage and a lobe to plug it.
“Amulet is the first and only minimally invasive treatment option to offer immediate closure of the [LAA], so blood-thinning medication isn't needed following implantation,” Abbott said in a press release. The postprocedural antithrombotic regimen following Watchman implantation, in contrast, requires a short bout of oral anticoagulation.
In addition, Abbott pointed out, Amulet “can treat a broad range of anatomies and has the widest range of occluder sizes on the market; it is also recapturable and repositionable to ensure optimal placement.”
Amulet has been available for several years in Canada, and Jacqueline Saw, MD (Vancouver General Hospital, Canada), whose center participated in the Amulet IDE trial, told TCTMD: “I’m really excited for my American colleagues now that they finally have more than one device. I think having the Amulet on the shelf is very important, because it does broaden the anatomy that can be closed percutaneously or from an endovascular approach.”
Having the Amulet on the shelf is very important, because it does broaden the anatomy that can be closed percutaneously or from an endovascular approach. Jacqueline Saw
Either Amulet or the Watchman FLX can close about 95% of all LAAs, Saw estimated, saying that having both available means that more than 99% of appendages can be occluded.
The results of the Amulet IDE trial will help clarify scenarios in which one device might be better than the other, Saw added. The Amulet, for instance, might have an advantage in terms of closing shallower appendages or those with sharper “chicken wing” bends, she said. It’s also possible that the Amulet, with its two layers of closure, will better minimize peridevice leaks, although that remains to be seen in the results of the Amulet IDE trial, she said.
An additional potential advantage of using the Amulet, Saw said, is the availability of a steerable sheath, which may help overcome a suboptimal transseptal puncture.
Nonetheless, both the Amulet and Watchman FLX are relatively straightforward to implant, and after overcoming the learning curve with a new device, “I think that operators can feel confident about implanting both devices pretty safely and efficiently once they’ve learned how to do it,” Saw said.
If the Amulet IDE trial shows comparable results in terms of hard outcomes and peridevice leak between the Amulet and Watchman devices, then operators will have the data needed to make a choice, Saw indicated. “If one anatomy is more suited to one device, then I would feel very confident to use that device, and if it’s suited for both devices according to anatomy, then I’ll be very confident to use either device.”
The Amulet has now been approved in more than 80 countries following its initial CE Mark approval in 2013, according to Abbott.
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Abbott. Abbott’s Amplatzer Amulet device approved by FDA to treat people with atrial fibrillation at risk of stroke. Published on: August 16, 2021. Accessed on: August 17, 2021.
Disclosures
- Saw reports unrestricted research grant support from the Canadian Institutes of Health Research, Heart & Stroke Foundation of Canada, National Institutes of Health, University of British Columbia Division of Cardiology, AstraZeneca, Abbott Vascular, Boston Scientific, and Servier; salary support from the Michael Smith Foundation of Health Research; speaker honoraria from AstraZeneca, Abbott Vascular, Boston Scientific, and Sunovion; consultancy and advisory board honoraria from AstraZeneca, Abbott Vascular, Boston Scientific, Baylis, Gore, FEops, and Abiomed; and proctorship honoraria from Abbott Vascular and Boston Scientific.



Comments