FDA Approves Lotus Edge, Ushering Third Device Into US Market
The decision marks the approval of the first fully repositionable and retrievable TAVR device in the United States.
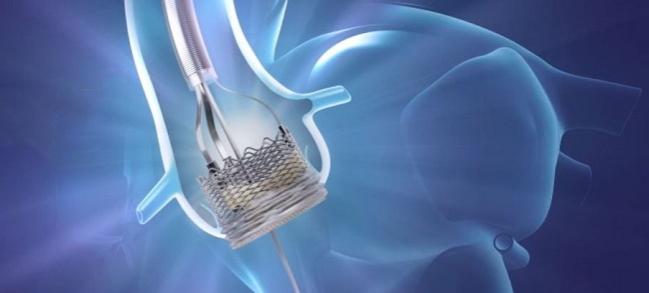
The US Food and Drug Administration has approved the Lotus Edge aortic valve system for transcatheter aortic valve replacement in patients at high risk for surgery, bringing—at long last—a third commercial player, Boston Scientific, into this space.
The approval, announced late yesterday, follows a “controlled launch” of Lotus Edge in Europe last month. The device was actually cleared in Europe back in September 2016, but later voluntarily withdrawn due to a faulty pin in the its delivery system.
Support for the FDA decision comes from the REPRISE III results, which featured the earlier-generation Lotus device but also included a nested registry of Lotus Edge recipients. REPRISE III results, presented at the EuroPCR 2017 meeting and reported by TCTMD, showed that the Lotus system was noninferior to a mixed population of CoreValve first-generation and Evolut R self-expanding valves (Medtronic).
Critics of the new valve have pointed out that the Lotus device has been associated with a high rate of pacemaker implantation—a problem the company has attempted to address with the innovation of a “depth guard” used during implantation. The guard allows for earlier anchoring and limits the depth of the implant to prevent interaction with the left ventricular outflow tract.
When 2-year outcomes for REPRISE III, published earlier this year, co-principal investigator Michael Reardon, MD (Houston Methodist DeBakey Heart and Vascular Center, TX), observed: “There are a lot of things that are pointing towards this being a really great third addition to the market. It's also still the only valve that can be fully deployed and then evaluated. Then if you like it you can release it, if you don't like it you can actually recapture it, remove it, change position.”
The other REPRISE III co-principal investigator, Ted Feldman, has since the joined up with the company that brought the first TAVR device into the US, Edwards Lifesciences, as Vice President, Medical Affairs, Transcatheter Mitral and Tricuspid Therapies.
Enrollment in the REPRISE IV trial, an intermediate-risk TAVR trial of Lotus Edge, began in early 2019, a company press release notes.
Approval may also increase the use of cerebral embolic protection during TAVR: Boston Scientific bought the Sentinel protection device in June 2018 and observers have frequently speculated that the plan would be to bundle the sale of these devices together.
Photo Credit: Adapted from Boston Scientific.
Shelley Wood was the Editor-in-Chief of TCTMD and the Editorial Director at the Cardiovascular Research Foundation (CRF) from October 2015…
Read Full BioSources
Boston Scientific. Boston Scientific receives FDA approval for LOTUS Edge aortic valve system. Published on: April 23, 2019. Accessed on: April 24, 2019.


Comments