FFR Pullback Measure Linked to Likelihood of Angina Relief After PCI
A low pullback pressure gradient indicates diffuse coronary disease that probably won’t respond well to stenting.
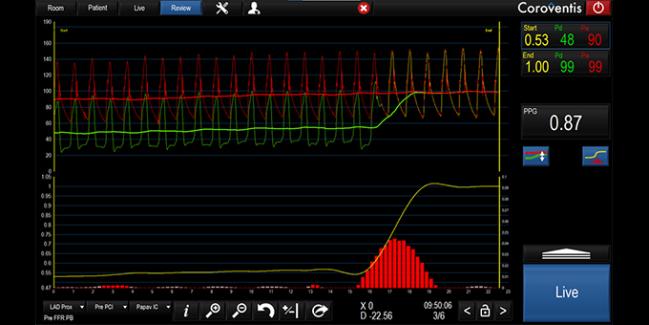
Vessel with an FFR of 0.53 and a pullback pressure gradient of 0.87, depicting focal disease (Photo Credit: Carlos Collet et al)
A novel metric derived from fractional flow reserve (FFR) pullback appears to be able to identify patients with coronary artery disease who are more likely to get symptom relief after PCI, which may help physicians decide who should or should not get a stent, data from a single-center study suggest.
The pullback pressure gradient (PPG), derived by pulling the FFR wire through the vessel to assess pressure changes prior to PCI, quantifies the pattern of coronary disease as focal or diffuse, standardizing what has been a subjective assessment.
In a post hoc analysis of the TARGET-FFR trial, patients determined to have diffuse disease according to PPG were roughly twice as likely to have residual angina after PCI as those deemed to have a focal lesion (51.9% vs 27.5%; P = 0.02). They also reported more physical limitations and worse quality of life.
To TCTMD, lead author Carlos Collet, MD, PhD (Cardiovascular Center, OLV Hospital, Aalst, Belgium), said the ability to use a standardized measure to define the presence of diffuse disease is “a big step for the field of coronary physiology,” as it removes “eyeballing” from the evaluation.
This has important implications for decision-making around management of patients with coronary disease, he indicated, noting that PPG is measured before stenting and thus may help identify patients who don’t require an intervention.
“We think that by integrating the classical FFR or other indexes with PPG . . . we’re actually providing a tool to refine the appropriateness criteria for PCI in terms of avoiding treatment of patients with diffuse disease or at least raising the awareness that diffuse disease is present and that the likelihood of improvement of that particular patient is low,” Collet said.
The findings were published online this week in in JACC: Cardiovascular Interventions.
Refining the Assessment of CAD Patterns
When FFR was developed 25 years ago, it was seen as a tool to define whether a lesion was significant or not, but researchers realized very quickly that the likelihood of improvement after PCI was more dependent on the pattern than the severity of disease, marking the beginning of the “pullback story,” Collet said. For the technique, after measuring FFR, instantaneous wave-free ratio (iFR), or other indexes, the operator simply pulls the wire back to assess the location of the pressure losses along the vessel, which takes only about 30 seconds.
The problem initially, Collet said, is that visual interpretation of the results from the pullback was subjective, with some operators seeing focal disease and others seeing diffuse disease. For that reason, Collet and his colleagues set out to develop a method to standardize interpretation a few years ago, and that effort culminated in the PPG, which is expressed as a number from 0 (indicating diffuse disease) to 1 (indicated focal disease).
To test whether PPG could be used to predict which patients are likely to get angina relief from PCI, the investigators performed a post hoc analysis of the TARGET-FFR trial of physiology-guided PCI, including 103 patients (mean age 60.6 years; 13.6% women) who had information available on PPG and scores of the 7-item Seattle Angina Questionnaire (SAQ-7) administered at baseline and 3 months after PCI.
Median PPG was 0.66. The 51 patients at this threshold or higher were considered to have focal disease, and the 52 below this cutoff were deemed to have diffuse disease. The two groups were similar before PCI, but after the procedure, patients with focal disease saw a larger increase in FFR (0.30 vs 0.19; P < 0.001).
We’re actually providing a tool to refine the appropriateness criteria for PCI in terms of avoiding treatment of patients with diffuse disease or at least raising the awareness that diffuse disease is present and that the likelihood of improvement of that particular patient is low. Carlos Collet
At baseline, angina frequency, physical limitations, and quality of life were statistically similar in the focal and diffuse groups, but differences emerged by 3 months. On average, SAQ-7 summary score was higher among patients who underwent PCI for focal disease (87.1 vs 75.6; P = 0.01), who also reported better mobility, self-care, and performance of usual activities, along with reduced pain and discomfort, on the EuroQol 5D-5L questionnaire.
The investigators also assessed the ability of PPG to identify angina-free status after PCI, calculating an area under the curve (AUC) of 0.65. Adding FFR and PPG to baseline clinical characteristics improved performance, with an AUC of 0.81.
“This finding highlights the importance of integrating CAD patterns derived from coronary physiology to define the appropriateness of PCI,” Collet et al write. “The quantitative and continuous nature of PPG allows the determination of cutoffs to predict improvements in angina, which may prove useful in clinical practice to predict the expected benefit of the intervention.”
Collet added that this is the first analysis “showing that quantifying the pattern of CAD as diffuse or focal with this PPG correlates with improvement in angina. Before this study, we did not know who will improve or not improve in angina.”
Impact on Clinical Decision-Making
Changes in FFR from before to after PCI have been correlated with the likelihood of angina improvement in prior research, but PPG gives physicians similar information before stenting has been performed, thus presenting an opportunity to defer interventions in patients who would benefit more from surgery or medical therapy, Collet noted.
Allen Jeremias, MD (St. Francis Hospital, Roslyn, NY), commenting for TCTMD, said this is an important study highlighting a need to change how physiology measurement is performed with FFR and iFR and go further to gain more insight into the distribution of the pressure losses. “The bottom line is by doing the pullback, now we actually can find a lot more information that was kind of hidden before when we just did a spot measurement in the distal vessel,” he said.
He agreed with Collet that this information can be used to guide treatment decisions. “When we do this pullback and we identify that we’re dealing with diffuse disease and not maybe a focal stenosis as the angiogram sometimes misleadingly indicates, you really have to change your strategy,” he said. “A lot of people may be resistant to that because obviously it’s a lot easier to just put [in] a focal stent and kind of ignore the rest of the information or not obtain the rest of the information. Now let’s say you do this pullback and you find diffuse disease, then obviously it’s much, much more difficult to treat, and I think one would realize that putting a focal stent into a diffusely diseased vessel is not going to solve the problem.”
That’s such an obvious finding that it might not be necessary to gather a lot more clinical data before applying it in practice, Jeremias indicated. “It’s more a matter of education and maybe also offering solutions,” which include medical therapy, more-extensive stenting of all areas of pressure loss in a vessel, CABG, or a hybrid procedure involving stenting of focal lesions in some vessels and bypass surgery in others, he said.
He added that he’s involved in the ongoing DEFINE GPS trial, which is comparing standard angiography-guided PCI with procedures guided by a system that uses measurements of pressure loss derived from iFR pullback. When diffuse disease is identified in the latter group, investigators are being asked to pursue a “very aggressive strategy” of stenting the entire segment of pressure loss.
The bottom line is by doing the pullback, now we actually can find a lot more information that was kind of hidden before when we just did a spot measurement in the distal vessel. Allen Jeremias
Collet et al acknowledge that prospective validation of the current PPG results is needed, along with further research to assess the impact on clinical outcomes of a PPG-guided PCI strategy.
The PPG Global Registry, which aims to enroll 1,000 stable patients and is assessing clinical and patient-reported outcomes, is ongoing, but what is being seen among investigators in the study is that they’re starting to defer stenting in vessels with a low PPG (below 0.30), Collet said. Procedural data will likely be reported next year.
“Beyond the finding of angina, what this technique brings to the field is that for the first time we have a standardized way to diagnose diffuse disease,” he explained, “and if this diffuseness of disease is associated with worse outcomes when treated with PCI”—as shown in another recently published paper from this group—"I think we will find a niche where PCI is even more effective, and I’m referring to the case of focal disease.”
While awaiting results of further studies and based on what’s known now, Jeremias said the takeaway is that “if somebody is committed to do a wire-based physiology assessment where you have a distal pressure measurement, then do a pullback also to get the information . . . and then really understand the distribution of pressure loss and understand better which patients should be treated and which potentially can be left alone, and don’t use the angiogram solely to make these decisions.”
In an accompanying editorial, Patrick Serruys, MD, PhD (National University of Ireland, Galway), and colleagues put PPG into the context of other techniques that have been developed over the years to assess the pathophysiology of CAD, saying that future trials comparing revascularization plus medical therapy with medical therapy alone “must include in their screening and protocol the assessment of focality and diffuseness as well as the detection of dysfunction of microvascular circulation.”
And patients with diffuse disease “without options for focal correction of intracoronary gradients may benefit from contemporary and future optimal medical therapy, which will no longer use only ‘symptomatic’ drugs (beta-blocker, calcium blocker, nitrate, and antihypertensives) but also powerful antiatherogenic drugs—for example, microRNA, anti–PCSK9 production, lipoprotein(a), high-sensitivity C-reactive protein, ligand against IL-6—which are able to reverse the diffuse atherosclerotic process.”
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Collet C, Collison D, Mizukami T, et al. Differential improvement in angina and health-related quality of life after percutaneous coronary interventions in focal and diffuse coronary artery disease. J Am Coll Cardiol Intv. 2022;Epub ahead of print.
Serruys PW, Kageyama S, Garg S, Onuma Y. In the beginning there was angina pectoris, at the end there was still angina pectoris. J Am Coll Cardiol Intv. 2022;Epub ahead of print.
Disclosures
- Collet reports receiving research grants from Biosensor, Coroventis Research, Medis Medical Imaging, Pie Medical Imaging, CathWorks, Boston Scientific, Siemens, HeartFlow, and Abbott Vascular; and having received consultancy fees from HeartFlow, OpSens, Abbott Vascular, and Philips Volcano.
- Jeremias reports consulting for several companies involved in coronary physiology technologies, including Abbott, ACIST, Boston Scientific, and Philips.
- Serruys reports having received institutional grants from Sinomedical Sciences Technology, SMT (Sahajanand Medical Technological), Philips/Volcano, Xeltis, and HeartFlow, outside the submitted work.



Comments