Heart Transplantation After Circulatory Death Gives ‘Encouraging’ Results
Compared with the traditional method of using hearts after brain death, this newer strategy could up the availability of organs.
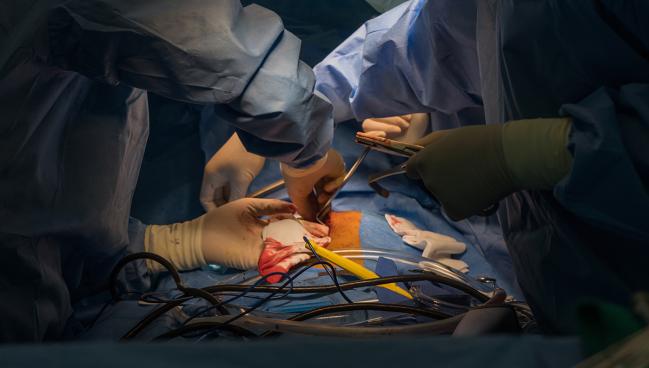
Heart transplantation using organs obtained from donors with circulatory death (DCD) is associated with transient right-heart dysfunction but offers short-term clinical outcomes otherwise comparable to surgeries using hearts from donors with brain death (DBD).
Surgeons have used DBD hearts for decades, but a shortage of donor organs has led to the transplant community to come up with other options. DCD hearts have emerged a potential solution, but ethical hurdles surrounding the confirmation of death in the donor are a point discussion, as well as challenges around preserving the hearts before transplant. New technologies including a proprietary warm-perfusion circuit (TransMedics), the organ care system (OCS) used in this study, as well as normothermic regional perfusion (NRP), have led to a handful of expert centers performing DCD heart transplant in the last few years.
“Early patient outcomes are encouraging in our initial single-center institutional experience with DCD heart transplantation using warm machine perfusion,” write David A. D’Alessandro, MD (Massachusetts General Hospital, Boston), and colleagues. “In our experience, this approach to DCD heart protection is effective, supporting the ongoing study of this practice as a predicate to broader adoption.”
Commenting on the study for TCTMD, Edward Soltesz, MD (Cleveland Clinic, OH), said he is “excited to see that the outcomes of the DCD hearts used with the TransMedics OCS system have shown equivalent results in the short term.”
He added: “This is going be an opportunity for us to continue to really look into this new method. The single biggest problem we have, of course, in heart transplant is just the availability of donor organs. So, I think this is certainly an opportunity to increase donor supply and also reduce wait times.”
Initial RV Dysfunction, Similar Outcomes
For the study, published in the October 4, 2022, issue of the Journal of the American College of Cardiology, D’Alessandro and colleagues included data from 47 DCD (mean age 55 years; 72.3% male) and 166 DBD (mean age 59 years; 71.7% male) heart transplants performed at their institution between April 2016 and February 2022. Notably, more patients in the DCD group were considered waiting-list status 6—meaning those who are stable enough to remain home while they wait for a heart—compared with the standard-of-care cohort (29.8% vs 3.0%).
While DBD hearts were transported stored in iced saline or using the Paragonix SherpaPak Cardiac Transport System, DCD hearts were harvested according to a newer technique. Generally, following a center-specific standoff period of about 5 minutes, the hearts were attached to the OCS. The organs were continuously monitored for aortic pressure and coronary flow, venous and arterial lactate levels, and visual appearance during transport. They were cooled on the circuit and then arrested with del Nido cardioplegia, removed from the OCS, and implanted using standard bicaval technique.
The median time from DCD consent to transplant was significantly shorter compared with those receiving standard of care (17 vs 70 days; P < 0.001). Following DCD transplant, there was a nonsignificant trend toward a higher incidence of severe primary graft dysfunction (PGD) at 24 hours compared with DBD transplant (10.6% vs 3.6%; P = 0.07). Also, venoarterial extracorporeal membrane oxygenation (ECMO) was required postoperatively for severe PGD in 10.6% of DCD recipients and 5.4% of DBD recipients (P = 0.20).
Notably, 1 week after transplant, DCD recipients had significantly impaired right-heart function compared with those who received DBD hearts, with higher median right atrial pressure (10 vs 7 mm Hg; P < 0.001), higher right atrial pressure to pulmonary capillary wedge pressure ratio (0.64 vs 0.57; P = 0.016), and lower pulmonary arterial pulsatility index (1.66 vs 2.52; P < 0.001). However, these outcomes no longer differed at 3 weeks.
All-cause mortality was similar in the DCD and DBD groups at both 30 days (0% vs 2%; P = 0.29) and 1 year (3% vs 8%; P = 0.16). Also, there were no differences between the groups in hospital length of stay following transplant, ICU length of stay, ICU readmission, or 30-day readmissions.
Researchers also observed improvements in the number of successful DCD procurements as center experience increased over the study period.
OCS vs NRP?
As for how to best preserve DCD hearts going forward, Soltesz said this remains unclear. “Over time, we'll learn potentially which is better for what type of a candidate,” he said. “The issue, of course, is in general we want to reduce wait time, we want to offer more hearts to people, but we have to be very careful in selecting which recipients are going to be appropriate for which type of heart.”
He said his institution performs DCD transplant with both the OCS method used in this study and NRP, with choice based on logistics. “It’s not only the donor organ that we have to look at and the way it's procured and which device or method we use to procure it, but also we have to try to match that with the patient,” Soltesz explained. “Meaning if we have a very sick patient in the hospital, they may not necessarily be the right candidate for one way of procuring the heart, for instance, compared to another.”
This was made clear in the study given how many more DCD recipients were considered status 6 compared with standard of care, for one, he pointed out.
Only a handful of centers in the US have been performing DCD transplants over about the last 4 years, Soltesz said. “I think you're going to be seeing a lot more centers starting to do them. I think that will be important, because we'll have a lot more data from various different centers, very different types of patients—both donors and recipients—to analyze.”
Comparing OCS and NRP in a trial, however, is unlikely to happen, he said, due to the “inherent bias” of the differences between the systems, Soltesz explained. “The problem is that the NRP method still has a limitation on distance of the cold ischemic time,” he said. “The TransMedics device allows us to go further, obviously, and have longer ischemic types because the heart's actually warm and beating. So unless, of course, we restrict the trial to certain lengths of transport and of ischemic time, I think it'll be unfortunately a biased trial.”
It’s likely that both methods will be needed for different purposes, he added.
Greater Standardization Needed
In an accompanying editorial, Ulrich P. Jorde, MD (Montefiore Einstein Heart and Vascular Center, Bronx, NY), writes that “it is clear that the use of hearts donated after circulatory death has become a significant part of our armamentarium and DCD using OCS is now widely accepted. As such, we can expect an increase in cardiac transplantation with estimates ranging from 10% to 25%, and this ultimately should lead to decreased wait times across the board.”
However, he acknowledges, “the process of donation after cardiac death as well as current technology to support it are likely to be substantially refined over the coming years. This may include extension of warm ischemic time, extension of time on OCS, mitigation of factors that lead to RV dysfunction, as well as severe PGD after DCD/OCS. Furthermore, the development of future OCS allowing comprehensive cardiac function testing may allow expansion of the acceptance criteria for DCD hearts.”
Jorde also points to the unsolved ethical issues of donation after cardiac death, “particularly when using NRP,” as something to watch going forward. “Central to this discussion is the definition of death and its irreversibility. In contrast to DBD, where brain death protocols are well established and accepted by societies across the globe, DCD protocol rules, eg, standoff times after complete cessation of circulation, continue to vary even within national jurisdictions,” he notes.
Soltesz, too, said he would like to see more standardization of both organ harvest and preservation across the country. “As we move along in this field of DCD transplantation, specifically with hearts, I think we're going to encounter a number of regulatory issues that we're going to have to deal with, hopefully at the national level, and define some national standards,” he said. “Right now, everything is sort of regional, and sometimes even within a single hospital they're able to define their protocols for DCD harvesting.”
Using the examples of how some hospitals now accept hearts from donors with hepatitis C as well as COVID-19, Soltesz said the research in this space is moving so quickly that “5 years from now, I think you're going to see a majority of the transplant programs across the country are going to be doing [DCD transplant].”
He would also like to see more research “go into understanding the other side of it, which is: how do you support patients waiting for a transplant? What's the best way to support them? Is unloading the ventricle with the transvalvular pump like the Impella pump important? We feel that it is, but is that critical? And how to manage PGD? We typically just put them on ECMO, but do we need to again add some form of unloading of the ventricle to optimize recovery?”
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
D’Alessandro DA, Wolfe SB, Osho AA, et al. Hemodynamic and clinical performance of hearts donated after circulatory death. J Am Coll Cardiol. 2022;80:1314-1326.
Jorde UP. Donation of hearts after circulatory death: a life saver. J Am Coll Cardiol. 2022;80:1327-1329.
Disclosures
- D’Alessandro and Soltesz report no relevant conflicts of interest.
- Jorde reports serving as a consultant for Abbott; and being an immediate past board member of the International Society for Heart and Lung Transplantation.



Comments