‘Landmark Moment’ for Middle Meningeal Artery Embolization for Subdural Hematoma
Three randomized trials supporting the approach will change practice in a fundamental way, Tudor Jovin says.
PHOENIX, AZ—Middle meningeal artery (MMA) embolization with liquid agents looks set to become the standard of care for the treatment of nonacute subdural hematoma after three randomized trials reported a week ago at the International Stroke Conference (ISC) demonstrated the procedure’s benefits.
All of the trials met their primary endpoints, showing significant reductions in hematoma recurrence/progression and other outcomes through 3 to 6 months of follow-up. Patients who were not treated first with surgical drainage appeared to derive greater benefits.
The release of these trials “represents a landmark moment in the treatment for this prevalent condition,” Tudor Jovin, MD (Cooper Neurological Institute, Camden, NJ), chair of ISC 2024, told TCTMD. “It’s going to change practice—I have no doubt about that—in a fundamental way. Endovascular treatment, embolization, will be the standard of care for treatment of subdural hematoma just like thrombectomy . . . became the standard of care for stroke due to large-vessel occlusion.”
Often caused by trauma, subdural hematoma is one of the most frequently encountered neurosurgical diseases, particularly in the elderly, and is associated with a high burden of morbidity and mortality. Due to the aging of the population and growing use of antithrombotic agents, it’s expected to become the most common cranial neurosurgical condition among adults by 2030.
Medical therapies for subdural hematoma generally have not proven to be very effective, and the main treatment is surgical drainage through a burr hole or small craniotomy. But recurrence rates are high—up to 20% in published series.
The problem, said Jason Davies, MD, PhD (University at Buffalo, NY), who presented the results of the EMBOLISE trial with Jared Knopman, MD (NewYork-Presbyterian/Weill Cornell Medicine, New York, NY), is that as blood and fluid collects between the surface of the brain and the overlying dura, membranes develop. The concept behind embolization of the MMA, which feeds that dural supply, he said, “is if we can devascularize these membranes by interrupting the blood vessels, we can dry up the membranes, interrupt that cycle, and hopefully reduce the recurrence rates and improve clinical outcomes for the patients.”
The EMBOLISE Trial
The first of the three trials presented at ISC, EMBOLISE, is an investigational device exemption (IDE) study of the Onyx liquid embolic system (Medtronic) for MMA embolization in patients with symptomatic subacute or chronic subdural hematoma. The trial consists of two separately powered cohorts—patients treated with surgical management (randomized to additional MMA embolization or surgery alone) and those not treated with surgery (randomized to MMA embolization or observation alone).
Davies and Knopman reported on the surgical cohort, which included 400 patients (mean age about 72; 73% men) enrolled across 39 sites. Overall, about 58% had chronic subdural hematoma, with the rest classified as having subacute disease. Type of surgical drainage was split roughly in half between burr hole evacuation and craniotomy.
This is a condition that affects a lot of people, particularly the elderly, with more and more patients on anticoagulation. Lauren Sansing
Embolization of the target vessel was successful in 100% of cases, with the presence of reflux to a nontarget vessel observed in only two cases.
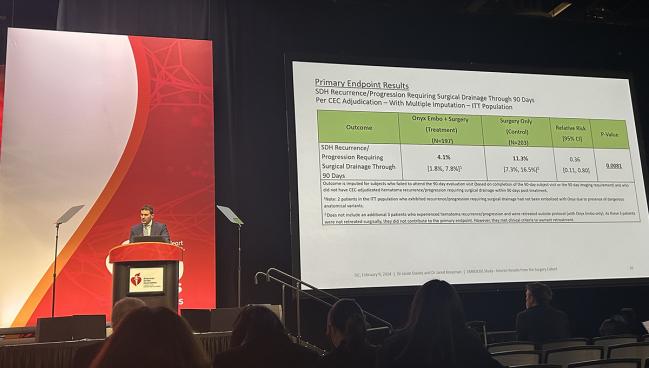
The primary endpoint was the rate of hematoma recurrence/progression requiring surgical drainage within 90 days of treatment, and this occurred in 4.1% of patients in the MMA embolization group and in 11.3% in the surgery-alone group (relative risk 0.36; 95% CI 0.11-0.80). That difference works out to a number needed to treat of about 14, Knopman said.
MMA embolization also proved to be noninferior to surgery alone for deterioration in neurologic function through 90 days.
In terms of safety, there was a low rate of serious adverse events related to MMA embolization within 30 days (2.0%), and there were no Onyx device-related adverse events through 90 days.
The rate of neurological death at 90 days was 4.6% in the embolization group and 2.0% in the control group (P = 0.17). Knopman noted that the vast majority of deaths were attributed to the subdural hematoma itself, with none determined to be related to the Onyx device or embolization procedure.
“We believe MMA embolization should be considered as an adjunct for patients who undergo surgical drainage of chronic and subacute subdural hematomas,” Knopman concluded. “We’re very excited by these results.”
MAGIC-MT
Next up, Ying Mao, MD, PhD (Huashan Hospital, Fudan University, Shanghai, China), presented results from the MAGIC-MT trial, conducted across 31 centers in 12 provinces in China. The study included 722 patients (median age about 69; 83% men) with symptomatic nonacute subdural hematoma who were treated with burr hole drainage (about 78% of the cohort) or conservative treatment. Within each group, participants were randomized to additional MMA embolization with Onyx or no embolization.
The primary endpoint was symptomatic recurrence/progression of subdural hematoma or death within 90 days, and this was reduced with MMA embolization (7.2% vs 12.2%; P = 0.02). Subgroup analyses suggested greater benefits in patients who were not treated with burr hole drainage, those with known head trauma, those with a midline shift no greater than 10 mm, and those with a smaller hematoma volume.
We’re very excited by these results. Jared Knopman
MMA embolization did not have a significant impact on functional outcomes, quality of life, the median length of the hospital stay, rehospitalization rate, or imaging outcomes. The rate of death at 90 days was numerically lower with embolization (0.6% vs 2.2%; P = 0.10).
Embolization was associated with a significantly lower rate of serious adverse events at 90 days (6.7% vs 11.6%; P = 0.02), with few complications related to the procedure.
These results, Mao said, need to be confirmed in additional trials.
The STEM Trial
Rounding out the trial presentations was Adam Arthur, MD (Semmes Murphey Clinic, Memphis, TN), who reported preliminary results from STEM, a study of MMA embolization using the Squid liquid agent (Balt) in patients with chronic subdural hematoma. The IDE study was conducted across 25 sites in the US and seven in Europe.
The trial included 310 patients (mean age 73.2; 69.6% men) randomized to standard management (either surgical or nonsurgical) or standard management plus MMA embolization. There were 189 patients in the surgical cohort and 121 in the nonsurgical cohort.
The primary endpoint was treatment failure, defined in three ways:
- Residual hematoma or reaccumulation of the hematoma (≥ 10 mm) at 180 days
- Reoperation after the index procedure or surgical rescue within 180 days
- Any new, major disabling stroke, MI, or death from any neurological cause within 180 days
The overall failure rate was 39.2% with standard management alone and 15.2% with adjunctive MMA embolization (OR 3.60; 95% CI 1.91-6.78). The difference appeared to be greater in the nonsurgical cohort than in the surgical cohort (OR 6.1 vs 2.4). “As you might expect for severely symptomatic patients with large subdurals, if you try to manage these patients conservatively, many of them will reach one of those three failure modes,” Arthur said.
The primary safety endpoints included major disabling stroke and any death within 30 days of the intervention. There was only one disabling stroke (in the control group), with a similar rate of all-cause death in the embolization and control arms (2.7% vs 3.1%). None of the deaths in the embolization group were attributed to the procedure or device, Arthur said.
Analysis of other endpoints is ongoing, he noted.
Growth of the Field
Lauren Sansing, MD (Yale School of Medicine, New Haven, CT), vice chair of ISC 2024, agreed that these three trials will be practice changing. “That’s going to make our endovascular colleagues much busier, I think, because this is a condition that affects a lot of people, particularly the elderly, with more and more patients on anticoagulation.”
Although MMA embolization is already being used in some cases, there hasn’t been class 1 evidence to support it, Sansing noted. But with these new results, the procedure is likely to become the standard of care, to be covered by insurance, and to reach more patients.
It’s going to change practice—I have no doubt about that—in a fundamental way. Tudor Jovin
Greater use of MMA embolization will have positive effects on the field of endovascular interventions for neurovascular conditions more broadly, Jovin suggested. “In general, it’s going to make endovascular therapy even more valuable, even more necessary, at hospitals, and . . . may lead to an increase in the need for endovascular practitioners.”
He noted that in recent years, there has been a push for more interventionalists to address the growing demand for mechanical thrombectomy in the setting of acute ischemic stroke. There hasn’t been as much for these new operators to do on the elective or semi-elective side, a gap that might be filled by MMA embolization in patients with subacute or chronic subdural hematoma, who don’t necessarily need immediate treatment.
“Now you have a procedure that will keep people busy even when they’re not on call,” Jovin said. “We need to have a healthy balance between elective and emergent procedures, and the subdural hematoma story is going to be very helpful for the growth of the field from that standpoint.”
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Davies J, Knopman J. EMBOLISE study: embolization of the middle meningeal artery with Onyx liquid embolic system in the treatment of subacute or chronic subdural hematoma. Presented at: ISC 2024. February 9, 2023. Phoenix, AZ.
Mao Y. Managing non-acute SDH using liquid materials: a Chinese randomized trial of MMA treatment (MAGIC-MT). Presented at: ISC 2024. February 9, 2023. Phoenix, AZ.
Fiorella D, Arthur A. STEM: Squid trial for the embolization of the middle meningeal artery for treatment of chronic subdural hematoma. Presented at: ISC 2024. February 9, 2023. Phoenix, AZ.
Disclosures
- EMBOLISE was sponsored by Medtronic.
- Davies reports receiving research grants from the National Institutes of Health, the National Science Foundation, the University at Buffalo Center for Assistive Technology, the Buffalo Translational Consortium, the Cummings Foundation, nVidia, and Google; having financial interests in QAS.ai, Rist Neurovascular, Cerebrotech, Synchron, and Hyperion; consulting or serving on the advisory board for Medtronic, Microvention, Imperative Care, Xenter, RapidPulse, Canon, and Johnson & Johnson; and serving as national PI or on the steering committee for StrokeNET DSMB, EMBOLISE, SUCCESS, RapidPulse, SBIR/STTR, and NIH NINDS/NLM Study sections.
- Knopman reports having financial interests in Hyperion and Serenity; serving as a consultant or proctor for Medtronic, Stryker, Microvention, Phenox, and Kaneka; and serving as national PI or on the steering committee for EMBOLISE.
- Mao reports funding from the Shanghai Shenkang Hospital Development Center, the Shanghai Municipal Health Commission, and Covidien/Medtronic.
- The STEM trial was sponsored by Balt.




Comments