Need for Rethink of Discharge Medications After LAA Closure? EMERGE
With no differences between single or dual antiplatelet therapy or anticoagulants at 6 months, an RCT is warranted for clarity.
WASHINGTON, DC—Single antiplatelet therapy may be a reasonable antithrombotic choice for some patients with atrial fibrillation who undergo left atrial appendage (LAA) occlusion, according to real-world data from the EMERGE LAA postapproval study.
Compared with patients discharged on dual antiplatelet therapy (DAPT) or oral anticoagulants, those sent home on either aspirin or a P2Y12 inhibitor following implantation with the Amplatzer Amulet (Abbott) had similarly low clinical event rates at 6 months, said Atman P. Shah, MD (University of Chicago, IL), in his presentation here at CRT 2025.
“I think the beauty of this is it allows us in these complex patients who come in for left atrial appendage closure to have the comfort that there is some data to personalize the type of therapy without incurring any increase in pericardial effusions, without increasing the risk of [device-related thrombus], or stroke,” Shah told TCTMD. “Speaking with the patient about this is absolutely critical because we know that patient compliance and patient adherence is very important. Adherence to oral anticoagulation is at best 80%, so . . . knowing options is important.”
The US Food and Drug Administration approved the Amplatzer Amulet device in 2021 based on the Amulet IDE trial, which showed better closure as well as noninferior safety and effectiveness compared with the first-generation Watchman device (Boston Scientific).
Current antithrombotic recommendations call for both aspirin and clopidogrel (or an alternate antiplatelet agent) for 6 months, with the decision to continue antiplatelet therapy, including aspirin alone, beyond then being at the discretion of the physician. Oral anticoagulation is primarily reserved for those in whom residual flow around the device after implantation is more than 5 mm.
Speaking with the patient about this is absolutely critical because we know that patient compliance and patient adherence is very important. Atman P. Shah
In a press conference prior to the presentation, Dean Kereiakes, MD (The Christ Hospital, Cincinnati, OH), pointed out that it would be helpful to have a breakdown of patients treated with aspirin versus those on a P2Y12 inhibitor given that, “in the arena of coronary heart disease and PCI, it's become very clear through multiple meta-analyses and studies like HOST-EXAM that P2Y12 monotherapy actually has more net clinical benefit than aspirin monotherapy.”
That analysis is in the works, Shah said, adding that the opposite observation seems to be true in the TAVI world.
“These are very exciting and hypothesis-generating issues and questions,” he added.
RCT Could Help Clarify Personalization
The EMERGE LAA postapproval study enrolled 11,445 patients (mean age 77 years; 40% women) who underwent successful Amulet implantation and were discharged between August 2021 and December 2023. Discharge medications included single antiplatelet therapy (SAPT) in 5.3%, DAPT in 81.7%, and an oral anticoagulant in 13%. Assignment to discharge medication was at the discretion of the operator.
More than 30% of patients in all three discharge groups had persistent or permanent atrial fibrillation and 20% had prior stroke. Patients in the SAPT group had the highest bleeding risk based on HAS-BLED score and bleeding history. More than 60% of patients discharged on SAPT remained on treatment through 6 months, as did 52.7% of those on DAPT and 23.7% on oral anticoagulants. Across all three groups, complete LAA closure was 84% or higher at 45 days.
At 6 months, the rate of the composite endpoint of all-cause death, stroke, major bleeding, or systemic embolism was 5.5% with SAPT, 6.9% with DAPT, and 4.9% with oral anticoagulants (P = 0.007).
Major bleeding was highest in the DAPT group at 3.8% compared with 3.1% with SAPT and 2.5% with oral anticoagulants (P = 0.022).
Clinical event rates for multiple individual outcomes were similarly low at 6 months between treatment groups (P = NS for all).
Six-Month Outcomes in LAA Closure Patients
|
|
SAPT |
DAPT |
Oral Anticoagulant |
|
All Stroke |
0.3% |
0.6% |
0.3% |
|
Systemic Embolism |
0 |
0.1% |
0.1% |
|
CV Death |
1.0% |
1.1% |
0.9% |
|
All-Cause Death |
2.5% |
3.0% |
2.6% |
|
TIA |
0.3% |
0.2% |
0.1% |
|
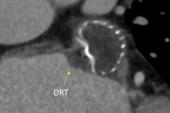
Device-Related Thrombus |
0.7% |
0.6% |
0.6% |
|
Pericardial Effusion |
0.8% |
1.1% |
1.2% |
David Cohen, MD (St. Francis Hospital & Heart Center, Roslyn, NY, and Cardiovascular Research Foundation, New York, NY), who moderated the press conference, said the study should spur more conversations on postdischarge therapies.
“It's a little bit embarrassing, frankly, that in the LAA field there hasn't been a good randomized trial of postprocedure management . . . [given that] the number of procedures that are being done is very large,” said Cohen.
That sentiment was echoed during the session by panelist Roxana Mehran, MD (Icahn School of Medicine at Mount Sinai, New York, NY).
“If left atrial appendage closure is going to be one of the most prominent procedures that we do because it is such a good procedure on so many levels for certain patients, why don't we know what we should be doing?” she said.
Panelist Nicolas Van Mieghem, MD, PhD (Thoraxcenter, Erasmus University Medical Center, Rotterdam, the Netherlands), said with no reimbursement for LAA closure in the Netherlands, the procedure is reserved for those with no other options and ongoing bleeding issues.
“I put in the LAA closure device and [prescribe] no antithrombotics at all, and I don't see an epidemic of strokes or TIAs,” he said told the media. ”We also do follow-ups by CT or by TEE at 45 days, but don't see the troubles. There is no good argument to continue the practice of DAPT, in my opinion.”
L.A. McKeown is a Senior Medical Journalist for TCTMD, the Section Editor of CV Team Forum, and Senior Medical…
Read Full BioSources
Shah AP. Outcomes of single antiplatelet, dual antiplatelet, or oral anticoagulation after Amulet: insights from the EMERGE LAA post-approval study. Presented at: CRT 2025. March 9, 2025. Washington, DC.
Disclosures
- Shah reports consulting for Abbott Vascular, Medtronic, and Boston Scientific.




Comments