New-Generation Watchman FLX Maintains Good Results Through 2 Years
Only one additional ischemic event was observed in the second year of follow-up, final results of PINNACLE FLX show.
MIAMI, FL—Occluding the left atrial appendage (LAA) with the next-generation Watchman FLX device (Boston Scientific) produces a low rate of stroke or systemic embolism through 2 years in patients with atrial fibrillation (AF) looking for an alternative to chronic oral anticoagulation, final results of the single-arm PINNACLE FLX study show.
One-year results, which were reported last year and supported US Food and Drug Administration approval, had demonstrated that the device provided effective closure of the appendage in all patients with few early adverse events, but a key question remained about what outcomes—particularly the risk of stroke—would look like after an additional year of follow-up.
At TVT 2021 held here last week, Saibal Kar, MD (Los Robles Health System, Los Angeles, CA), one of the principal investigators, reported that the 2-year rate of stroke or systemic embolism was 3.4%, coming in significantly below the performance goal of 8.7%, which was based on the expected rate of 4.0% derived from prior Watchman studies “plus a clinically relevant delta.” All but one of those events occurred in the first year of the study.
There were no cases of device embolization through 2 years of follow-up, and no new cases of device-related thrombus (DRT) detected in the second year.
“We observed a sustained safety and efficacy outcome through 2 years,” Kar concluded, saying later during a panel discussion that the low rate of safety events “is very encouraging both for patients and physicians.”
Commenting for TCTMD, Poonam Velagapudi, MD (University of Nebraska Medical Center, Omaha), who was not involved in the study, said the Watchman FLX “had really good 2-year outcomes” in these patients with AF who had an indication for oral anticoagulation but also had a reason to seek a nonpharmacological alternative, such as a history of bleeding. “Overall, the results were what I was expecting, and obviously the device performs better than the older-generation Watchman device.”
Velagapudi said advantages of the newer iteration include a wider range of sizes, two layers of anchors, and a closed distal end; it’s also shorter. There are “multiple safety features in the new device that help it to anchor and cause less trauma to the appendage,” she explained.
During the panel discussion, Mark Reisman, MD (Weill Cornell Medicine, New York, NY), said “the difference between the original 2.5 and the FLX is a significant difference and a tremendous improvement in the safety, usage, comfort, and even the malleability of the device within the appendage. . . . This seems to be really a fairly significant change in the right direction.”
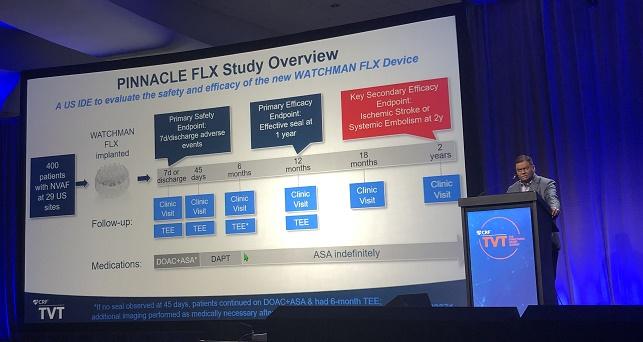
PINNACLE FLX
PINNACLE FLX, the US investigational device exemption trial, enrolled 400 patients with AF (mean age 74 years; 36% women) at 29 US sites. Mean CHA2DS2-VASc score was 4.2, mean HAS-BLED score was 2.0, and 33% of patients had prior major bleeding or a predisposition to bleeding. All patients were imaged with transesophageal echocardiography (TEE) at 45 days, 6 months (if no seal was observed at 45 days), and 1 year. All other TEEs were performed in response to events, Kar said.
As previously reported, the Watchman FLX provided an effective seal at 1 year in all patients, with a low rate of early adverse events (0.5% at 7 days/discharge). At 1 year, the rate of stroke or systemic embolism was 3.1%, and only one additional ischemic event was recorded in the second year of follow-up.
A variety of adverse clinical outcomes were less frequent in the second versus first year of the study. Though 2 years, 9.3% of patients died from any cause, 5.5% died from CV causes, 3.4% had any type of stroke, 3.1% had an ischemic stroke, 0.3% had a hemorrhagic stroke, and 0.3% had a systemic embolism.
The rate of major bleeding (BARC type 3 or 5) was 8.2% in the first year and 1.9% in the second, with all of the bleeds in the second half of the study classified as BARC 3.
The rate of pericardial effusion requiring surgery or pericardiocentesis was 1% overall, with all events occurring in the first year.
All seven DRT cases (1.8%) occurred in the first year—four in patients who were taking dual antiplatelet therapy and three in patients who were taking aspirin alone. Two of these people developed ischemic stroke or systemic embolism, including one who died.
Take DRT Findings With ‘Pinch of Salt’
In a panel discussion following Kar’s presentation, Rebecca Hahn, MD (NewYork-Presbyterian/Columbia University Irving Medical Center, New York, NY), questioned the validity of reporting no DRT cases in the second year of follow-up when TEEs were not routinely performed during that phase. “It seems as though imaging was rather sparse in the follow-up patient population,” she said.
Kar acknowledged that there could have been more if they had looked for them, but said no other Watchman trials had mandated follow-up TEEs in all patients beyond 1 year.
Asked about the issue, Velagapudi noted that findings from a LAA occlusion registry recently published in the Journal of the American College of Cardiology showed that 20% of DRTs were detected more than a year after the procedure.
“I think it’s really important that we take [the PINNACLE FLX finding] with a pinch of salt since we didn’t have routine imaging,” she said, adding, however, that the low rate seen in the study could be related to the modifications made to the newer device. “I think this is a good finding, but we should be a little bit wary and aware of the fact that they did not have routine TEE imaging.”
That limitation aside, Velagapudi said “there’s a huge population of patients that could benefit from left atrial appendage closure when they’re not able to take these anticoagulants.
She said she wouldn’t comment on use of LAA occlusion in patients who are good candidates for either oral anticoagulation or appendage closure, as that population is currently being studied in the CHAMPION-AF trial (Kar is one of the principal investigators). “But right now, I think the group of patients that it’s really helpful [in] would be those who have a high CHA2DS2-VASc score, greater than 2 in men and greater than 3 in females, who cannot tolerate anticoagulation or are unable to maintain their therapeutic INRs [on warfarin],” Velagapudi suggested.
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Kar S. First report of 2-year outcomes with a next-generation left atrial appendage closure device: final results from the PINNACLE FLX IDE trial. Presented at: TVT 2021. July 21, 2021. Miami, FL.
Disclosures
- Kar reports receiving grant/research support from Abbott Vascular, Boston Scientific, Edwards Lifesciences, Medtronic, and HiLife; receiving consulting fees/honoraria from Abbott Vascular, Boston Scientific, Medtronic, and W.L. Gore & Associates; and serving as the principal investigator of trials for Abbott Vascular and Boston Scientific.
- Velagapudi reports no relevant conflicts of interest.



Comments