Novel Repositionable TAVR Device Sees Low Pacemaker Rates and Few Leaks
Thirty-day results with the Centera valve hint that it may be possible to overcome both of TAVR’s Achilles’ heels, experts say.
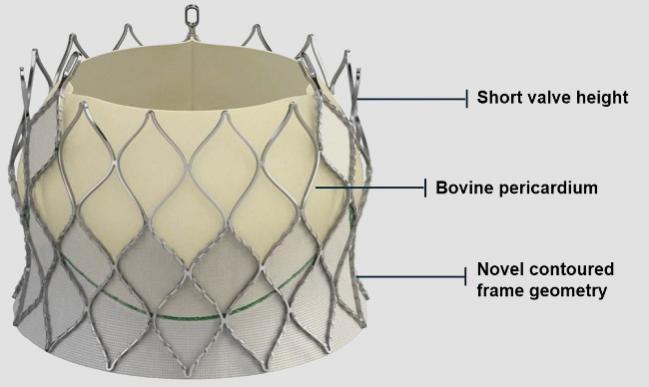
Thirty-day results seen in 203 patients treated with a self-expanding, repositionable, transcatheter valve in patients with severe, symptomatic aortic stenosis at high surgical risk suggest that this latest-generation device is safe and effective, researchers say.
The Centera device (Edwards Lifesciences)—used at 23 centers in Europe, Australia, and New Zealand in the nonrandomized CENTERA-2 study—was associated with low rates of both paravalvular leak (PVL) and permanent pacemaker implantation (PPI). Hermann Reichenspurner, MD (University Heart Center, Hamburg, Germany), and colleagues reported their findings online December 18, 2017, in the Journal of the American College of Cardiology.
Centera’s system includes a valve made of a contoured, self-expanding, nitinol frame and bovine pericardial tissue leaflets preattached to a 14-Fr-compatible, motorized delivery system that allows the device to be repositioned. Three sizes of the device were studied (23 mm, 26 mm, and 29 mm), and delivery was transfemoral.
An earlier feasibility study in 15 patients pointed to favorable short- and mid-term clinical outcomes and improved hemodynamics.
CENTERA-2 at 30 Days
In the current series, mean age of the as-treated cohort was 82.7 years, 67.5% of patients were female, 68% were NYHA class III/IV at baseline, mean STS score was 6.1, and mean logistic EuroSCORE was 17.1. Successful implantation was achieved in 198 of 203 patients. Of the five untreated patients, two had procedure-related deaths—one patient had excessive vascular access calcification and bleeding followed by Sapien 3 (Edwards Lifesciences) implantation and another had guidewire-related tamponade. The remaining three patients experienced valve embolizations and were subsequently treated with surgery or Sapien 3.
By 30 days in the as-treated group, 1% of patients had died, disabling stroke had occurred in 2.5%, and NYHA functional class I/II was seen in 93%. Hemodynamic parameters also improved significantly from baseline. Of note, paravalvular aortic regurgitation was moderate or higher in just 0.6% of patients at 30 days, with no cases of severe leak; new pacemakers were implanted in just 4.5% of patients.
The pacemaker rate is “exceptionally low,” the authors note, pointing out that other self-expanding valve platforms have reported rates between 11.7% and 26.3%, while balloon expandable devices have seen pacemaker rates ranging from 6.5% to 12%. “Potential explanations for the low observed permanent pacemaker rate may be the related to the low frame height of 18 to 23 mm . . . and the minimal protrusion in the left ventricular outflow tract,” the authors write.
‘Very Competitive’
Commenting on the CENTERA-2 results for TCTMD, Josep Rodés-Cabau, MD (Quebec Heart and Lung Institute/Laval University, Canada), who clarified that he has never used the device, called the results “very competitive” with those seen with other transcatheter valves already on the market in the United States and Europe. In terms of valve performance, these results with the Centera—the largest series published to date, he noted—suggest that it could be used “in the majority of patients, with very good results.”
Like the authors themselves, Rodés-Cabau zeroed in on the low number of new pacemaker implantations, which he called “really good” and said, in his opinion, can likely be explained by a range of factors. These include the motorized delivery system, permitting repositioning, the shorter valve frame, and potentially a slightly lower radial force at the level of the left ventricular outflow tract. In addition, the judicious, wait-and-see approach taken by investigators to see if conduction disturbances resolved on their own may also have helped reduce the rate of permanent pacemaker implantations.
“So there’s a mix of things that make a difference: the valve properties, very good positioning, and probably a good management of conduction disturbance,” he said. The valve sizing was also very well done, Rodés-Cabau added, in that the study used a centralized core lab for cardiac CT annular sizing and for all echocardiographic measurements.
In an accompanying editorial, Luis Nombela-Franco, MD (Instituto de Investigación Sanitaria del Hospital Clínico San Carlos, Madrid, Spain), makes the point that other devices have tended to make progress either in limiting paravalvular leaks or in reducing the need for a new pacemaker, but seldom both together.
“Although TAVR has demonstrated similar or even lower rates of mortality, stroke, major bleeding, acute kidney injury, and new-onset atrial fibrillation compared to surgery, PVL and PPI have been considered the Achilles’ heel of TAVR,” Nombela-Franco comments. “Some of the key mechanistic factors in these two complications may be inversely related, and improving one factor may jeopardize the other.”
He continues: “Although we must be cautious in the absence of direct randomized comparisons and larger series, it seems that this valve could overcome previous weaknesses of [transcatheter heart valves] and compete against surgery in terms of the PPI and PVL rates. If we are able to reduce significant PVL and PPI rates to 1% and 5%, respectively, this valve would represent a major step forward in the TAVR horizon with clinical improvement for our patients.
Rodés-Cabau agreed. “We will have to see how this valve performs in the real world with no inclusion criteria, but the results are very, very promising,” he told TCTMD. “I think this study provides the rationale to say this valve is ready to compete.” However, he said, longer follow-up and larger patient numbers are needed, a point also made by the study authors. CE Mark is expected for the Centera in Europe either before the end of 2017, or in early 2018. A US pivotal trial is in the works for 2018.
Photo Credit: Tchetche D. The Self-Expanding Single-Operator CENTERA TAVR System. Presented at: TCT 2017. October 31, 2017. Denver, CO.
Shelley Wood was the Editor-in-Chief of TCTMD and the Editorial Director at the Cardiovascular Research Foundation (CRF) from October 2015…
Read Full BioSources
Reichenspurner H, Schaefer A, Schäfer U, et al. Self-expanding transcatheter aortic valve system for symptomatic high-risk patients with severe aortic stenosis. J Am Coll Cardiol. 2017;70:3127-3136.
Nombela-Franco L. New valves may overcome weaknesses of transcatheter aortic valve replacement. J Am Coll Cardiol. 2017;70:3137-3139.
Disclosures
- Reichenspurner reports receiving speaker support and travel honoraria from Edwards Lifesciences and serving as a consultant to HeartWare/Medtronic.
- Nombela-Franco reports serving as a proctor for Abbott and receiving speaker honoraria from Edwards Lifesciences Inc.


Comments