Radiation Exposure Highest for Interventional Imagers at Right Waist and Below
With no standard shielding used for imagers in structural heart procedures, several experts are calling for a consensus document.
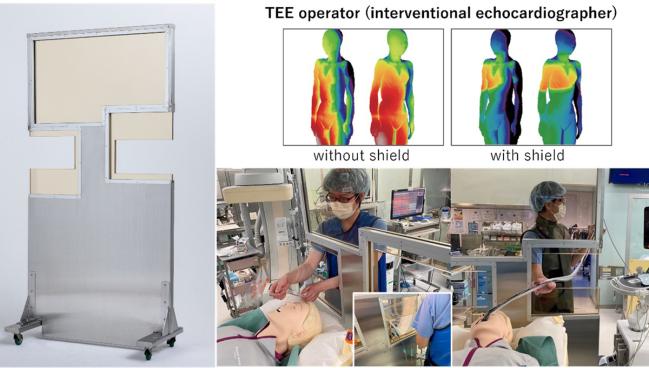
New shielding for the imager, with ergonomic cutouts, now used at the researchers’ institution (Photo Credit: Akihisa Kataoka)
For interventional imagers participating in structural heart interventions, new simulation data shows high radiation exposure on the right side of the body, especially near the waist and below, with variations depending on procedure type.
Still a nascent field, interventional imaging has grown in both size and scope, with prior research highlighting the occupational dangers associated with this work, especially for those performing TEE procedures.
Despite ongoing education efforts by leaders and societies, the awareness of radiation exposure risks and mitigation of them “are still very low” among individual physicians, lead author Akihisa Kataoka, MD, PhD (Teikyo University, Tokyo, Japan), told TCTMD in an email. This is of particular importance to female practitioners, given that many of them are of childbearing age, he said. “Many female physicians and female healthcare professionals work in medical settings worldwide, and raising awareness about this issue among medical staff is an urgent issue that directly relates to the diversity of the workplace and the ability of female physicians to work with peace of mind.”
No standardization currently exists for cath labs to install shielding or other protection measures for interventional echocardiographers, although these are generally in place for interventional cardiologists and anesthesiologists alike.
This became painfully clear for Kataoka during the COVID-19 pandemic, when he began watching structural heart intervention demonstrations virtually. “I was surprised to see numerous institutions appear to be indifferent to radiation protection,” he said. “Therefore, I believe that many heart teams are not aware of the radiation exposure issue.”
Similarly, João L. Cavalcante, MD (Minneapolis Heart Institute Foundation, MN), who commented on the study for TCTMD, said tools like ceiling-mounted lead shields are standard in most US cath labs for the operator but not on the left side of the table for the echocardiographer. Frequently, Cavalcante said, he hears of imagers having to borrow shielding from the anesthesiologist. He urged the imagers themselves to speak out.
“They have to be vocal; they cannot be shy,” he said. “They have developed and devoted skills that are to be laudable, and we cannot perform the advances of structural imaging without their eyes, without their guidance. And the hospitals need to recognize that actually the return on investment is immense. It would not be felt immediately, but for sure it would be felt when a hazard or cancer or cataract or other things comes up.”
As well as being a “mandatory piece” of the training curriculum for an interventional imager, radiation safety should also be at the forefront of job discussions for these physicians, Cavalcante added. “They need to look and visit the cath lab,” he said. “They need to see what resources are there, and if [proper shielding] is not there, that should be part of the negotiation to make sure that they are protected.”
Simulation and Clinical Practice
For the study, published online today in JACC: Asia, Kataoka and colleagues first conducted a simulation to estimate the radiation dose on the body surface of interventional echocardiographers performing TEE in a hybrid cardiac surgery suite. Additionally, they measured the clinical dose of radiation exposure to interventional echocardiographers performing 79 procedures—44 transcatheter edge-to-edge repairs (TEERs) using MitraClip (Abbott) and 35 TAVRs—in a retrospective, observational data set from 2021.
The simulation identified high-dose radiation exposure (>20 mGy/h) to the right side of the imager’s body, especially at the waist and below. This was primarily due to scatter radiation from the bottom of the patient’s bed. These high-doses of exposure were present for obtaining both posterior-anterior and cusp-overlap views.
Also, the simulation estimates were consistent with what was observed in the clinical cohort. In the latter, TEER led to more radiation exposure at the interventional imager’s waist compared with TAVR (median 0.334 mSv/mGy vs 0.053 mSv/mGy; P < 0.001), as did TAVR with self-expanding compared with balloon-expandable valves (median 0.067 mSv/mGy vs 0.039 mSv/mGy; P < 0.01).
Kataoka said he was most surprised that the cusp-overlap view, which is currently recommended for self-expanding TAVR procedures, had the highest absorbed dose rates in all positions during their simulation, but especially high at the echocardiographer’s waist. “[This] is a technique that is highly praised for its effectiveness by renowned catheter operators and TAVR manufacturers, but the fact that it poses a significant risk of radiation exposure to echocardiographers, particularly young women, has not been adequately addressed,” he said. “I believe that this goes against social ethics and, as a result, many young female echocardiographers are likely being exposed to radiation unnecessarily.”
Standardization Needed
In an accompanying editorial, Kevin Ka-Ho Kam, MBChB, and Alex Pui-Wai Lee, MBChB, MD (both Prince of Wales Hospital, The Chinese University of Hong Kong, China), write that the study findings provide further grounds for a “consensus document or guideline addressing radiation safety [to] be established as soon as possible.”
Until then, they recommend the “installation of dedicated lead shields, minimal use of right anterior oblique fluoroscopic views, and close observation of the ‘as low as reasonably achievable’ (ALARA) principle” to reduce radiation exposure to interventional echocardiographers.
However, they note some limitations of the study. Primarily, Kam and Lee say, because the simulation performed did not involve any regularly used shielding including a lead apron, neck shield, and goggles, which are “universally worn in all kinds of cardiac intervention procedures,” the amount of radiation exposure calculated will undoubtedly be higher than in clinical practice.
Kataoka acknowledged that “unfortunately, there was no ideal lead shield protection installed at the head end of the procedure table where the interventional echocardiographer would stand.” They had tried to install a large conventional freestanding protective plate between the interventional echocardiographer and the patient, but “this made the interventional echocardiographer’s and the anesthesiologist's work difficult,” he said, noting that the procedures in this study were performed without this shield. However, they have since developed and begun using a new shield with “ergonomically optimized cutouts on both sides” to allow for productivity and protection.
Additionally, Kam and Lee write, because TAVR practice has changed so that most patients are imaged via a transthoracic approach, the radiation exposure for these procedures has dropped well below what is still seen with TEER.
“The comparison of left atrial appendage occlusion to TEER would be more appropriate in view of the necessity of continuous echocardiographic guidance and monitoring of complications,” the editorialists suggest.
“Nonetheless, I think the message is that the investment in the cath lab procedure for the interventional cardiologist needs to be the same, and if even not more, for the interventional echocardiographer,” Cavalcante said. “Procedures now require team participation. The team needs to be protected.”
The addition of a RADPAD (Worldwide Innovations and Technologies), a disposable radiation shield placed on the patient’s midsection to intercept scatter radiation, is also a useful practice, according to Cavalcante. “The other message that I would like to highlight is when we think about ways to decrease radiation exposure, the three main factors are how much you are pressing on the fluoroscopic pedal, so the magnitude of the total radiation exposure; the shielding; but also the distance from the radiation exposure,” he said.
An impactful avenue for future development might be longer TEE probes that place the imager farther from the radiation source, he suggested.
Kam and Lee would also like to see studies on a variety of radiation protective measures and shields specific to interventional echocardiographers. “As the cardiology community, we need to continue to raise awareness of radiation safety, to recognize the expertise of structural heart disease imagers, and to protect and nurture young-generation cardiologists who are interested in this field,” they stress.
Imagers are exposed to this risk for the duration of their careers, “and the amount of cumulative radiation exposure is substantial,” Cavalcante added. “Particularly as newer procedures are being introduced, . . . they are going to be exposed until that procedure matures, catheters are developed, et cetera.”
He urged societies and hospitals to “come up with the very bare minimum for a workplace.” Ergonomics are frequently discussed in this setting, “but also equally important is the radiation exposure that they need to be trained on. They need to have a refresher, and demand that cath labs provide the adequate support,” Cavalcante urged.
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Kataoka A, Takata T, Yanagawa A, et al. Body surface radiation exposure in interventional echocardiographers during structural heart disease procedures. J Am Coll Cardiol Asia. 2023;Epub ahead of print.
Kam KK-H, Lee AP-W. Radiation exposure of interventional echocardiographers protecting and nurturing a new subspecialty. J Am Coll Cardiol Asia. 2023;Epub ahead of print.
Disclosures
- This study was supported by the Terumo Life Science Foundation, Japan 2020 R&D subsidies, the Abbott Medical Japan LLC scholarship fund, the Boston Scientific Co scholarship fund, the Japan Society for the Promotion of Science, and the Japan Science and Technology Agency ERATO Grant.
- Kataoka reports receiving remuneration from Abbott Medical Japan as a proctor for MitraClip.
- Lee reports serving as a consultant for Abbott, Pfizer, Huihe, Dawneo, Shenqi, Philips, and Fujifilm and receiving research grants from Sanofi, Bayer, Novartis, Boehringer Ingelheim, Jessen, and AstraZeneca.
- Cavalcante and Kam report no relevant conflicts of interest.




Comments