Realistic Expectations Emerge After Initial Excitement Over Xenotransplant
Physicians and innovators point to cost, immunosuppression, and genetic engineering challenges, but they remain hopeful.

The acute success of the first xenotransplantation using a genetically engineered porcine heart into a man with end-stage heart disease 2 weeks ago had many cardiologists and cardiac surgeons excited about what seems to be an abrupt paradigm shift. But as time goes on, some are urging humility and warning against overhyping this relatively unproven technique.
The procedure, conducted in 57-year-old David Bennett at the University of Maryland Medical Center (UMMC), was authorized by the US Food and Drug Administration under its compassionate use provision given that Bennett was not eligible for a human heart transplant or an artificial ventricular assist device. He remains in stable condition and continues to recover, according to a UMMC source.
Upon hearing the news of the surgery, heart failure specialist Douglas Mann, MD (Washington University School of Medicine in St. Louis, MN), came to realize how much progress had been made in recent years, he told TCTMD, and was reminded of a quote by heart transplant pioneer Norman Shumway, MD: “Xenotransplantation is the future, and always will be.”
“After something like this, there's going to be this huge swell of enthusiasm and you'll have everybody and their uncle getting into the xenotransplant business or be excited about it,” said Mann. “And then the reality of it kind of dampens people's spirits a little bit, because maybe [in] other places they'll get acute rejection or patients won't do so well.”
We have to do it thoughtfully and correctly in order to prove that this is a good thing for patients, rather than just sort of all of a sudden jumping onto the bandwagon. Douglas Mann
Pediatric cardiovascular surgeon David Cleveland, MD (University of Alabama at Birmingham), who has participated in research looking at heart transplants from pigs to infant baboons, urged that “you have to be a little bit humble here” because much remains unknown. “I think this is a great achievement,” he commented to TCTMD. “I think it's a really important step forward. I'm very hopeful that this patient will live for months, and hopefully years. There's potential to go that long.”
Mann also hailed the UMMC transplant as “a huge step forward.” Excitement is warranted, he agreed, “but we also ought to realize how much more work we have to do, and we have to do it thoughtfully and correctly in order to prove that this is a good thing for patients rather than just sort of all of a sudden jumping onto the bandwagon.”
The Bennett case represents “a real triumph of science and genetic engineering,” continued Mann, who stressed, though, that animal organs won’t suddenly appear on the market as “pig hearts for everyone.”
“There are a lot of issues that have to be worked through,” he said.
Decades of Genetic Research
One of the biggest technological breakthroughs that has allowed for the resurgence of xenotransplant is gene editing. In 1964, Boyd Rush was the first man to undergo xenotransplant, receiving a chimpanzee heart that beat for 90 minutes, and in 1984, infant Stephanie Fae Beauclair (often referred to as Baby Fae) famously lived for 21 days after receiving a baboon heart. Yet scientists were decades away from being able to sequence the human genome, much less edit it with CRISPR-Cas9.
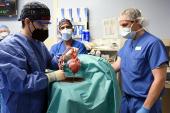
The heart used in Bennett’s operation was manufactured by a company called Revivicor (Blacksburg, VA), owned by United Therapeutics. The heart had a total of 10 genetic modifications, including four knockouts—three responsible for rapid antibody-mediated rejection of pig organ and one to prevent excessive growth—and six human genes added to increase likelihood of acceptance. The company declined to comment for this article but confirmed they raised their genetically modified pigs in several locations, including Virginia and Alabama.
Cardiac surgeon Bruno Reichart, MD, PhD (LMU Klinikum, Munich, Germany), who serves as CEO of the xenotransplant company XTransplant, called the heart used at UMMC a “humanized organ.” Working in conjunction with Revivicor, his team contributed toward the invention of the knockout that limits growth of the pig heart, he told TCTMD.
Additionally, Reichart and colleagues helped develop what they call nonischemic heart protection to sustain the explanted organ before transplantation. “When we cut out the porcine heart, we put it immediately on the pump, on a little heart-lung machine with a low pressure, and the pump cools the heart to 8 centigrade,” he explained. In pictures published by UMMC, the donor heart can be seen removed from the XVIVO Heart Box, which uses the process Reichart describes.
This has the potential to offer another paradigm shift in the way we approach single-ventricle heart disease or critical congenital heart disease. David Cleveland
Michael Curtis, PhD, president of research and development at eGenesis (Cambridge, MA), a company doing similar work, told TCTMD they are currently focused on building a genetic engineering platform that focuses on three goals: “inactivating retroviruses, getting rid of antigens in the genome that are responsible for hyperacute rejection, and then adding human transgenes to promote long-term graft survival.”
A relative newcomer to the field of xenotransplant, eGenesis primarily uses CRISPR-Cas9 to develop their donor pig cells and somatic cell nuclear transfer—the same process that was used with Dolly the sheep—to clone them, Curtis explained. They don’t yet have a breeding herd of pigs to use for potential organ donation, but that is their end goal.
While eGenesis also includes the six human transgenes in its pigs, “we only do three knockouts because we're working in what's called a Yucatan mini pig, so it's a smaller pig,” he said. “The Revivicor team is working in large white pigs, and they have to deal with the heart overgrowing the recipient. So to limit the size of the organ, they make this growth hormone receptor knockout. . . . We don't have to do that.”
Working with the Yucatan mini pig holds the advantage in terms of size, but these animals have a higher likelihood of the porcine retrovirus PERV-C, which could be dangerous if passed onto humans. “We go right at it and try to robustly inactivate retroviruses using CRISPR-Cas9,” while Revivicor hearts innately should be PERV-C-free, Curtis said. “Those are different philosophies of how to address the retrovirus transmission risk. We'll see which one is actually the best.”
Kidney transplant is the main focus for eGenesis right now, having conducted about 30 nonhuman primate transplants last year, Curtis said. They are not yet gearing up to get into the clinic with a heart, but given the success “that Maryland hopefully will have,” the company may ramp up their plans, he said.
Cleveland said that while the Revivicor heart with its specific genetic modifications seems to have done well so far, it’s not yet clear what specific genetic engineering will be required for a perfect match. “I think it's a shot in the dark to be quite honest,” he said. “People are guessing.”
But Is It Affordable?
A big hurdle for the future of xenotransplantation, as with any new technology, is cost.
Nature reported that each porcine transplant into a baboon cost approximately $500,000. That is approximately fivefold higher than what’s seen for a human heart transplant, Mann estimated. “And that's just the acute cost,” he said, adding that follow-up myocardial biopsies and immunosuppressives are expensive as well. “This could be a fantastically expensive technology that the country can't afford.”
Greater experience, efficiency, and competition should lower the total cost, Mann said, “but initially it's going to be expensive.”
Neither Revivicor nor UMMC would comment on the total cost of either the pig heart or the total procedure. eGenesis, as well, would not comment on the total cost of producing a donor animal, though they said in an email that “once there is a breeding herd (rather than cloned donors), the cost will drop significantly.”
Curtis also believes competition will be a good thing for the field. “I think we all have a slightly different take on the approach to engineering, the approach to producing the pig,” he said. “We are all always in contact with each other, and if there is a collaboration that would make sense, there's no reason to recreate the wheel.”
Cleveland agreed, suggesting that “so many possible iterations” may exist for the optimal donor pig. It’s entirely possible that the current Revivicor product “may not be the best pig out there,” he said, adding that competition in the marketplace is important.
’Working Against Yourself’
In addition to cost, the question of immunosuppressive therapy poses another challenge in the field of xenotransplant.
“We always start with full-court press as far as immunosuppression, because we're just not sure how much we can rely on the transgenes that we put into these organs,” Curtis explained. “You always start with as much immunosuppression as you think is reasonable from a safety perspective and see how it goes. What we often do in our primate studies is if we get out past 100 days, then we can start to back off.”
Immunosuppression comes with its own risks though. As has been seen with kidney transplant, “the immunosuppression actually compromises the transplanted organ in many cases,” said Curtis. “You are kind of working against yourself.” The ultimate goal is to “get to a place where you can engineer the organ where you would have minimum immunosuppression and get to long-term survival,” he continued. “Then you can start to open that up to a larger population.”
[The goal is to] get to a place where you can engineer the organ where you would have minimum immunosuppression and get to long-term survival. Michael Curtis
In the case of Bennett, Mann said he would expect the UMMC team to use heavy immunosuppression for at least a year, if not longer.
Curtis believes the issue of acute rejection to have been solved, yet added that there are multiple ways a xenotransplant can fail early that are beyond rejection. “If you make it to 100, 200 days posttransplant, it's a positive predictor of what you can expect clinically,” he said. “[That would] definitely buoy the field, but if it were not to go long, let's say over 30 days or something, we'd have to look really closely at why.”
In this way, single patient studies are “dangerous,” Curtis continued, “because we could have a negative result which has maybe nothing to do with rejection . . . that could mislead us.”
Mann would like to see more compassionate use cases—done in patients who, like Bennett, have no other options—to show continued success. Then, following those, a randomized clinical trial would ideally show longevity. Curtis said that single, investigator-sponsored, emergency use studies are a steppingstone for the field, but only a clinical study with multiple patients will demonstrate that results can be replicated.
A ‘Renewable Resource of Organs’?
Ultimately, “the best way to prevent somebody from needing a heart is to do the hard work of treating the diabetes, the blood pressure, putting them on the right medicines,” Mann said, adding that this is where the focus should stay. “That's the cure to prevent it from happening rather than putting in these expensive technologies when everything else has failed.”
For Curtis, envisioning a world with a “renewable resource of organs” for those who need them is not out of the realm of possibility. “All of this science is really coming together to turn xenotransplantation into some version of reality, and I think we're at a point now where we have made significant enough advancements that we can ask the question: are we going to have a meaningful impact on patients who suffer from end-stage renal disease or chronic heart disease?”
“There's been a huge amount of progress made,” Cleveland said. And while there’s a long way still to go, “this has the potential to offer another paradigm shift in the way we approach single-ventricle heart disease or critical congenital heart disease.”
Cleveland said he is most optimistic about the potential for xenotransplantation in infants on the waiting list for heart transplants, especially those with single ventricle anatomy who aren’t good candidates for mechanical circulatory support. “The latest results suggest that it's about a 50% mortality at 6 months,” he said. “So that's a pretty low bar to jump over.”
His team is pursuing the idea of using xenotransplantation as a bridge to allotransplantation—using a human heart when an organ becomes available—but so far they have only done four experiments in baboons with only two surviving long-term: one for 3 months and the other for 8 months.
“We have babies dying all the time in our intensive care unit that have no real option, and I think it's worth a try if we could get an exemption,” Cleveland said. “I was surprised that the FDA gave their permission to do it with the amount of data [there is], but it's encouraging to me that they did. I thought ok, let's open the door now.”
Note: An earlier version of this story incorrectly identified Baby Fae as the first xenotransplantation patient.
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioDisclosures
- Mann reports no relevant conflicts of interest.
- Cleveland reports receiving two pigs for research purposes from Revivicor but no monetary support.


Shelley Wood
ROBERT DERVELOY