Reduced Leaflet Motion After TAVR: No Link to Impaired Hemodynamics, Events
The reduced motion was largely relegated to just one leaflet and resolved irrespective of oral anticoagulant use in 10% of cases.
Reduced leaflet motion (RLM), predominantly affecting one leaflet, is observed in approximately 15% of patients with aortic stenosis treated with a transcatheter heart valve, although less-severe hypoattenuated leaflet thickening (HALT) affects roughly one-third of patients after TAVR, according to a new analysis from the PORTICO investigational device exemption (IDE) study.
Although the study was not powered for hard clinical endpoints, there was no relationships between RLM and the risk death, stroke, or any other outcome. Nor was there any association between RLM and valve hemodynamics. Interestingly, RLM appeared to resolve spontaneously irrespective of oral anticoagulant use.
“Regression is a real phenomenon in around 9% to 10% of patients whether or not they were on oral anticoagulation,” said lead investigator Hasan Jilaihawi, MD (NYU Langone Medical Center, New York, NY), during a late-breaking clinical science presentation earlier this week at TVT Connect.
Julinda Mehilli, MD (Ludwig-Maximilians University, Munich, Germany), one of the discussants during the session, described the new study’s results as challenging to understand. For example, she cited the lack of correlation between RLM and impaired valve hemodynamics.
“You have reduced leaflet motion, but there’s no hemodynamic impact on the valve,” she said. “These are very asymptomatic patients, so the mechanism as to why we see this is difficult to understand. Also, what are the characteristics of patients who developed RLM? Are they younger? Do they have other comorbidities? Is there something special in these patients compared with the rest of the group?”
Regression is a real phenomenon in around 9% to 10% of patients whether or not they were on oral anticoagulation. Hasan Jilaihawi
Samir Kapadia, MD (Cleveland Clinic, OH), another discussant, pointed out that valve degeneration resulting from impaired hemodynamics is a late phenomenon, and while studies have not conclusively shown that RLM or HALT leads to higher rates of stroke or death, he doesn’t think the phenomenon can be ignored from a research perspective. For him, as well as the other experts, the long-term implications of the RLM and HALT are still not clear.
“The durability of the valve: the question is definitely not solved,” said Kapadia.
Jilaihawi agreed that the vast majority of patients were asymptomatic and had only one leaflet with reduced motion, which may account for the hemodynamic findings. “This was over a relatively short time frame,” he said. “When we’re talking about durability, we certainly need to look at least over 5 years, and probably even more. It’s possible we might start to see some changes over that time point.”
With respect to risk factors for RLM, Jilaihawi pointed out that this was a high/extreme-risk group and that STS-PROM score was a predictor of reduced motion. In multivariate-adjusted regression analysis, use of oral anticoagulation also was associated with a lower risk of RLM at 30 days, as was the native aortic valve area.
First Report in 2015
Subclinical leaflet thrombosis was first documented in the PORTICO IDE trial in 2015 when Raj Makkar, MD (Cedars-Sinai Medical Center, Los Angeles), and colleagues identified reduced aortic-valve leaflet motion on CT in patients treated with the Portico valve (Abbott), as well as other commercially available valves including surgical bioprostheses. Several registries and prospective studies have since evaluated the incidence of reduced leaflet motion, including RESOLVE and SAVORY as well as industry-sponsored low-risk CT substudies, and shown similar findings.
Following the findings from PORTICO IDE, researchers launched a prospective study nested within the trial to evaluate the incidence of RLM and its impact on clinical outcomes and valve hemodynamics. They also sought to compare the frequency of RLM by valve brand.
The PORTICO trial included high and extreme surgical risk patients with severe symptomatic aortic stenosis treated with the Portico valve or various iterations of Sapien (Edwards Lifesciences) or CoreValve (Medtronic). The nested substudy included 321 patients—167 treated with the Portico device and 154 treated with other transcatheter valves—who mostly underwent CT scans (3-D transesophageal echocardiography [TEE] was used in rare cases) at 30 days. At 6 months, the investigators had 238 analyzable CT/TEE scans.
At 30 days and 6 months, respectively, RLM was observed in 15.9% and 15.5% of all patients who received a transcatheter heart valve. In an exploratory analysis, the incidence of RLM among patients who received the Portico valve was 24.6% and 19.5% at 30 days and 6 months, respectively. With the Sapien valve, the incidence was 6.9% and 11.8% at the same time points. For those who received CoreValve, it was 5.8% and 8.8%, respectively. With all valve types, only one leaflet was negatively impacted in the majority of RLM cases. In the entire cohort, HALT was observed in one-third of patients at 30 days and 6 months, and it also predominantly affected one leaflet.
When looking at the longitudinal and natural course of RLM—the natural course excluded patients who were treated with oral anticoagulation—more than 71% of patients without RLM at 30 days had no evidence of RLM at 6 months. There was a progression from no RLM at 30 days to RLM at 6 months in 6.3% of all subjects and 8.2% of those not on oral anticoagulation. Impaired motion was persistent from 30 days to 6 months in roughly 10% of patients, while regression was observed in 9.7% of all patients and 9.4% of subjects not on oral anticoagulation.
The durability of the valve: the question is definitely not solved. Samir Kapadia
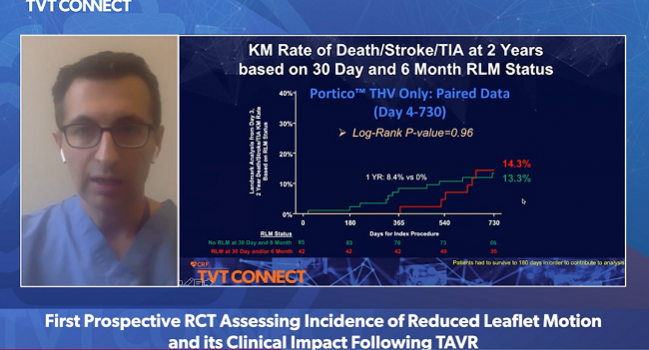
In an analysis that excluded procedural complications and focused on clinical outcomes from 4 to 180 days, there was no significant difference in any hard endpoints at 6 months between those with and without RLM at 30 days. There also was no difference in clinical outcomes among those with and without HALT at 30 days. In Kaplan-Meier analysis, the risk of death, stroke, or transient ischemic attack at 2 years was 13.9% in patients with no RLM at 30 days and 6 months and 15.4% in patients with RLM at 30 days and/or 6 months, a nonsignificant difference.
With respect to hemodynamics, there was no difference in the mean transvalvular gradient among any of the patient groups, including those with persistent RLM from 30 days to 6 months or those who had a progression of RLM. By valve type, there was no significant difference in the mean gradient among those with and without RLM.
Not Powered For Clinical Endpoints
In the discussion, Jilaihawi stressed that the study was not powered to draw any conclusions regarding RLM and clinical outcomes, valve performance, or differences between transcatheter heart valves.
Session moderator Stephan Windecker, MD (Bern University Hospital, Switzerland), asked what clinicians should do when they observe RLM clinically, noting that regression appeared to occur as frequently in those who did receive oral anticoagulation as those who didn’t. Also, he wondered, should clinicians even look for it?
Kapadia said they don’t routinely screen for HALT or RLM. They only refer patients for CT if they detect an increase in the transvalvular gradient, in which case they will prescribe oral anticoagulation if impaired leaflet motion is observed. Mehilli said they practice the same approach in their clinic. Six months after TAVR, if HALT is detected by CT but there are no changes in transvalvular gradient, they will follow the patient more closely. Oral anticoagulation is only prescribed if there is a change in gradient on TEE in conjunction with CT findings.
Michael O’Riordan is the Managing Editor for TCTMD. He completed his undergraduate degrees at Queen’s University in Kingston, ON, and…
Read Full BioSources
Jilaihawi H, on behalf of the PORTICO IDE investigators. First prospective randomized, controlled trial assessing incidence of reduced leaflet motion and its clinical impact following TAVR. Presented on: June 21, 2020. TVT Connect 2020.
Disclosures
- Jilaihawi reports grant/research support and/or consulting/honoraria/or speaking fees from Abbott Vascular, Medtronic, Edwards Lifesciences, Boston Scientific, and Venus Medtech.
- Mehilli reports grant support from Boston Scientific, AstraZeneca, Bristol-Myers Squibb, Medtronic, and Edwards Lifesciences.
- Kapadia reports equity/stock options in Navigate and Admedus.
- Windecker reports grant support and/or research contracts with Abbott Vascular, Amgen, BMS, Bayer, Boston Scientific, Biotronik, Cardinal Health, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Johnson & Johnson, Medtronic, Guerbert, Polares, Sanofi, and Terumo.


Comments