Registry Results See Less PVL, Pacemakers With Acurate neo2
Ahead of the pivotal IDE trial, non-US registry data for this newer-generation TAVI device raise hopes it has left its troubles behind.

LONDON, England—Observational postmarketing surveillance data for the next-generation Acurate neo2 (Boston Scientific) suggest this latest TAVI device, engineered to mitigate the risks of paravalvular leak (PVL) seen with an earlier iteration, is associated with very low rates of mortality, disabling stroke, PVL, and new pacemaker implantation at 30 days.
Of note, however, 4D-CT imaging, measured by a core lab at 30 days, suggested that nearly one in four patients had some degree of hypoattenuated leaflet thickening (HALT), a finding that investigators in this field have yet to fully understand.
The postmarketing observations for Acurate neo2 come in the wake of the SCOPE I and SCOPE 2 randomized trials, in which the earlier-generation device proved inferior to Sapien 3 (Edwards Lifesciences) and CoreValve Evolut (Medtronic), respectively. In both trials, the investigational valve was also associated with rates of moderate or severe PVL in the range of 10%.
As reported by TCTMD, an earlier glimpse at Acurate neo2 performance in a registry already provided some reassurances that the newer-generation valve had cut down on the worrisome PVL rates seen with the earlier iteration. The pivotal US investigational device exemption (IDE) trial now underway is using the newer device, which was granted European clearance back in 2020.
Won-Keun Kim, MD (Kerckhoff-Klinik, Bad Nauheim, Germany), presented results from the prospective, multicenter, postmarketing clinical follow-up registry here during a late-breaking trial session at PCR London Valves. They also were published simultaneously in EuroIntervention.
Speaking with TCTMD, Kim said the results point to the potential for the device to take a place among other approved TAVI valves.
“I'm a big fan of patient-specific valve selection, and I don't think that currently there's one valve that covers all aspects when you take into account patient specific anatomy, comorbidities, et cetera,” he said, listing the range of factors required to manage aortic valve stenosis over a patient’s lifetime. “The Acurate neo2 . . . has most of the ingredients that are necessary for lifetime management.”
Thirty-Day Results
Kim presented results for 250 patients enrolled at 18 centers participating in this postmarketing registry (mean age 80.8 years; 63.6% female). In all, 246 were successfully treated with the device; four patients (1.6%) experienced device embolization, caused by oversizing in one instance and malpositioning in the others.
At 30 days, the primary safety endpoint of all-cause mortality was seen in two patients (0.8%)—one died from hemodynamic instability of unknown cause and the other from device embolization followed by an unsuccessful TAV-in-TAV rescue. Two nondisabling and zero disabling strokes occurred within the first month.
New pacemakers were implanted in 6.5%, a rate that Kim, speaking with TCTMD, characterized as “the lowest rate seen among contemporary devices.” Moreover, this was achieved without having to “aim for a high position,” which can cause problems for coronary access, he said.
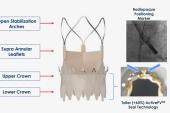
Indeed, these are some of the reasons why he thinks the US market still has room for this latest addition, should the pivotal trial prove positive. In addition to the low pacemaker rates and a supra-annular position, the device also allows for commissural alignment, further preserving coronary access, said Kim. Moreover, “for lifetime management, you need to have good gradients, and the Acurate neo is a supra-annular valve with 1-digit gradients. This will play a role in the future for long-term durability.”
Leaflet Mobility
The primary imaging endpoint, HALT measured by a central core lab, was detected in 24.5% of patients, with HALT > 50% seen in 9.3% of patients. In roughly half of the patients with some degree of HALT, investigators also saw evidence of restricted leaflet mobility.
Kim explained to TCTMD that the imaging endpoint was required by regulators, but said the rate of 25% is not unexpected. In PARTNER 3, the rate was nearly 30% at 1 year, he noted, and that was a lower-risk population than patients treated in this registry.
“Despite the relatively high incidents of HALT [in these registry findings] there was no signal for any harm and the stroke rate was very low,” he said. “To see how this develops over time, we will do 1-year follow up with CT, but I have no idea actually [whether it will prove clinically meaningful]. We need to have this imaging, because we have to learn, but currently nobody knows how to really evaluate those findings on CT and we have no data supporting that we have to do a full cycle CT in every patient, especially if patients are getting younger.”
Making PROGRESS?
Following Kim’s presentation, panelist Andreas Ruck, MD, PhD (Karolinska University, Stockholm, Sweden), asked about the four device embolizations, calling this “surprising” and adding that “it's a bit more than we've seen in other studies with the Acurate.”
Kim, in response, called it “a bit of bad luck,” although he noted that the rate of device embolization for other self-expanding devices tends to be in the range of 1.5%. Operator inexperience may have played a role here, he speculated, pointing out that the rate of embolization in other Acurate neo2 registries has been lower.
Panelist Antoinette Neylon, MD (Hôpital Privé Jacques Cartier, Massy, France), queried whether the new, taller sealing skirt has materially improved the valve performance. “In practical terms, how much better does it change the type of anatomy that we can use this technology in?” she asked.
The final session question may have been the one on everyone’s mind. “Bottom line,” asked Chaim Lotan, MD (Hadassah-Hebrew University, Jerusalem, Israel), “would you say that this is noninferior or similar to what we have in the market? Or maybe even superior?”
Kim hedged, calling this a “difficult” and “political” question. “I would say we have to try to get the best valve for each patient and there are different valves with different characteristics,” he said.
Kim may have more-detailed answers after the IDE trial wraps up. “Trial enrollment is ongoing, and I think they have reached a good level,” he told TCTMD. “I’m hopeful that enrollment will be completed maybe in Q1, Q2 next year, and then this will be very interesting in terms of showing comparison with the contemporary devices that are available in the US, [particularly] looking back at SCOPE I and 2.”
Shelley Wood was the Editor-in-Chief of TCTMD and the Editorial Director at the Cardiovascular Research Foundation (CRF) from October 2015…
Read Full BioSources
Kim WK, Tamburino C, Möllmann H, et al. Clinical outcomes of the ACURATE neo2 transcatheter heart valve: a prospective, multicenter, observational, post-market surveillance study. EuroIntervention. 2022;Epub ahead or print.
Disclosures
- Kim reports receiving personal fees from Boston Scientific, Edwards Lifesciences, Abbott, Medtronic, and Meril.




Comments