Tendyne at 2 Years: Confirmed Feasibility but High Mortality With TMVR
Patient selection has stalled progress of transcatheter MV replacement: low survival implies further refinement is needed.

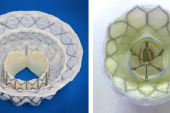
Photo Credit: Muller DWM. TENDYNE: 1-year outcomes of transcatheter MV replacement in patients with severe mitral regurgitation. Presented at: TCT 2017.
Yet two in five people treated died during follow-up, most within the first 3 months, leading some to insist that patient selection needs to be further refined before this procedure can be used more routinely.
Progress in the space of transcatheter mitral valve treatment has been slow going compared with advancements in aortic valve disease. Three years ago, the COAPT trial made waves showing the success of transcatheter edge-to-edge mitral valve repair using the MitraClip (Abbott) in patients with heart failure and severe functional MR, but not all anatomies are suited to this procedure. Prior to that, 1-year findings from the Tendyne Global Feasibility Study were published for the first 30 patients implanted with the device, a trileaflet porcine pericardial valve that is delivered transapically and permanently anchored to the apex with a tether. Encouraging 30-day data in the first 100 patients came in 2018, and the device was granted CE Mark approval in early 2020.
“For the transcatheter mitral valves, it has been difficult to identify the right population of patients to treat, and it's also a much more difficult valve to work with,” lead author David Muller, MBBS, MD (St Vincent’s Hospital, Sydney, Australia), told TCTMD. “There have been a number of devices that haven't made the grade, if you will, and have been discontinued. Tendyne is the only valve that has actually done well in the commercial sense. There are now more than 800 patients who have been treated with this device.”
The 2-year findings of the same first 100 patients (mean age 74.7 years; 69% male) are now published in the November 9, 2021, issue of the Journal of the American College of Cardiology and confirm what was observed at 1 year. Heart failure hospitalization was less frequent than it had been preprocedure (0.51 vs 1.30 events per year; P < 0.0001), 93.2% of surviving patients had no MR at 2 years, and none had > 1+ MR. The 2-year rate of all-cause mortality remained high at 39.0%, with 43.6% of deaths occurring in the first 90 days.
For survivors, the device appeared to improve symptoms: 88.5% of patients were in NYHA functional class I or II at 1 year, a proportion that dipped only slightly, to 81.6%, at 2 years. From baseline to 2 years, reductions in both LVEF (45.6% to 39.8%; P = 0.0012) and estimated right ventricular systolic pressure (mean 47.6 to 32.5 mm Hg; P < 0.005) were seen among survivors.
“It's clear that this procedure does carry some risk in the initial postoperative period, but for patients who survive that period, and particularly those who are still doing well at 1 year, the outcomes beyond that are excellent,” Muller said. “So we're really very pleased about that.”
Patient Selection Is Key
Commenting on the study for TCTMD, Firas Zahr, MD (Oregon Health and Science University, Portland), said he was reassured to finally see intermediate-term data that “renew trust” in the therapy. “The field of TMVR—with a big R [for replacement]—has been a little bit challenged by our ability to identify the right patient population for this therapy,” he said. “The 90-day mortality rate tells us that despite a highly selective group of patients who pass screen fail, we still need a lot of work to do to understand which patient population will benefit the most from this therapy. However, it seems like [for] the patients who are surviving at 2 years, their functional class, their heart failure hospitalization, their quality of life is much better.”
In an accompanying editorial, Josep Rodés-Cabau, MD, PhD (Quebec Heart & Lung Institute, Quebec City, Canada), and colleagues focus on the need for better patient selection, what they deem an “unsolved issue” in the field. Echoing Zahr’s comments, they note that the screening failure rate in TMVR trials has ranged from 40%-70%. “So, it is concerning that despite these highly selective criteria, the risk of treatment futility within the months following the procedure seems to be high, likely due to the high comorbidity burden of current TMVR candidates,” they stress.
Muller said that most of the patients who seem optimal for TMVR with Tendyne will be elderly, with poor heart function and plenty of comorbidities. “But if we take those who are too elderly and too frail, then we may fix their valve, but they'll die from other causes and from poor heart function,” he said. The same was seen in early TAVI studies, he noted, where limiting procedures to the sickest patients inevitably led to worse outcomes. Over the next few years, he predicted, the field will increasingly turn to “a population of patients perhaps who are younger and who are not as frail, who can survive the initial surgery.”
The upcoming SUMMIT trial will include patients who are eligible for both MitraClip and TMVR and will give “a better sense I think of whether this can compete with MitraClip or whether those who are anatomically eligible for MitraClip should have that and only the patients who just are not suitable for that will be considered then for this procedure,” Muller continued.
The editorialists also call out the “negative impact of the transapical access in a frail population with left ventricular dysfunction.” Switching from a transapical to a transseptal approach might reduce recovery time and morbidity as well as access-related myocardial injury, they say. “Unfortunately, due to the current design of the Tendyne valve, the ability to switch from transapical to transseptal implantation with this platform is highly unlikely.”
This is probably true, Muller confirms. “There's been a lot of work from a lot of companies trying to develop a similar concept that can be deployed as a transeptal procedure, . . . but I would have to say that no company has really got that one sorted yet,” he said, adding that with the Tendyne valve specifically, the importance of the tether’s effect on outcomes remains unknown. “From an engineering point of view, it can be very hard to create a device that is tethered to the apex from a transeptal approach.”
Rodés-Cabau and colleagues also discuss the balance of bleeding and valve thrombotic risk among patients who undergo TMVR as “one of the main challenges” of the procedure. “Of note, there were no cases of valve thrombosis during the second postoperative year, suggesting that relatively short periods of aggressive antithrombotic therapy may be the strategy with the best risk/benefit ratio in these patients,” they write. “Further studies are urgently needed to determine the optimal type and duration of antithrombotic therapy in this field.”
In a JACC podcast summarizing the study, Editor-in-Chief Valentin Fuster MD, PhD (Icahn School of Medicine at Mount Sinai, New York, NY), said while the study has “good news, intermediate news, and bad news,” the evidence is not convincing enough yet to see a definite clinical use of this procedure given available medicines. “In my view, right or wrong, my approach would be just trying to improve symptoms,” especially given the difficulty of life following any potential procedure-related complications he said.
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Muller DWM, Sorajja P, Duncan A, et al. 2-year outcomes of transcatheter mitral valve replacement in patients with severe symptomatic mitral regurgitation. J Am Coll Cardiol. 2021;78:1847-1859.
Rodés-Cabau J, Regueiro A, Mack MJ. Transcatheter mitral valve replacement: a need for better patient selection. J Am Coll Cardiol. 2021;78:1860-1862.
Disclosures
- The present paper is an analysis of the first 100 patients treated in the Expanded Clinical Study of the Tendyne Mitral Valve System (Global Feasibility Study; NCT02321514) supported by Abbott.
- Muller reports serving as a consultant for Medtronic, Abbott, and Edwards Lifesciences; and receiving research grant support from Abbott and Medtronic.
- Rodés-Cabau reports receiving institutional research grants from Edwards Lifesciences and Medtronic and holding the Research Chair “Fondation Famille Jacques Larivière” for the Development of Structural Heart Disease Interventions.
- Zahr reports no relevant conflicts of interest.



Comments