Pace Quickens for Mitral Device Innovation for Repair, Replacement
A EuroPCR 2018 session dedicated to treatment of mitral regurgitation demonstrates how much this challenging field has advanced.
PARIS, France—Interventions for the treatment of severe mitral regurgitation has advanced to the point where investigators are able to dive deeper into complicated clinical issues and learn from studies of larger populations, as evidenced by several studies presented here at EuroPCR 2018.
Commenting to TCTMD, Ted Feldman, MD (Evanston Hospital, IL), who chaired the ‘hotline’ session, said, “the whole mitral valve replacement field is moving. We had a long period where enrollment in these various registries and trials was really, really slow, and now we're seeing triple digit numbers of patients being reported in single device and trial efforts. So, the ability to learn from our experience with these numbers is palpable.”
Talking Tendyne
Presenting first, David W. M. Muller, MBBS, MD (St. Vincent’s Hospital, Sydney, Australia), showed 30-day outcomes for the first 100 patients enrolled in the CE Mark study of the Tendyne tri-leaflet porcine pericardial valve (Tendyne/Abbott). As reported by TCTMD, 1-year data from the global feasibility study showed improved NYHA functional class, reduced patient symptoms, and a decrease in mitral regurgitation (MR).
The device consists of a self-expanding nitinol double frame which contours to fit the mitral annulus. It is delivered transapically and a tether is secured to a pad at the bottom of the heart to hold the valve in place permanently.
Ninety percent of patients enrolled in this study had secondary or mixed MR at baseline with a grade of 4 or higher and all were deemed poor candidates for surgery. Technical success was achieved in 97%, with the implant needing to be retrieved in two patients due to LV outflow tract (LVOT) obstruction and an inability to position the device correctly, and abandoned in one patient due to pulmonary edema. There were no periprocedural deaths, strokes, or emergency surgeries and no need for extracorporeal membrane oxygenation. Six patients died by 30 days—four from cardiorespiratory failure, one from hospital-acquired pneumonia, and one from sudden unexpected death.
Among the 75 patients with full 30-day data, all patients reported none/trace MR except for one who had grade 1+ MR. One patient had a stroke, eight patients were rehospitalized for heart failure, three had pleural effusions, nine had bleeding requiring transfusion, and four had new or worsening renal failure. Only two patients reported paravalvular leak.
At 30 days, the proportion of patients who were classified as NYHA class III or IV dropped from 62.5% at baseline to 24.9% (P < 0.0001), and the mean Kansas City Cardiomyopathy Questionnaire score improved from 50.5 to 58.0 (P = 0.037).
Lastly, echocardiography at 30 days showed improvements in LV end-diastolic volume (P < 0.0001), LV end-systolic volume (P = 0.027), and LVEF (P = 0.0005).
“We would conclude from this that this is a very predictable procedure that is well tolerated hemodynamically,” Muller concluded. The team is expecting to enroll the first patient in SUMMIT, the US pivotal trial, by this fall, he reported.
During the Q&A following Muller’s presentation, Feldman questioned both the apical tether’s effect on ventricular performance outcomes as well as the “screening funnel” used for this study in terms of including or excluding patients.
Muller replied that while the tether does play a role and that he “used to think it was a liability,” his team has started to investigate it deeper and found “some very intriguing changes that happen immediately after the valve is implanted that then correct themselves very quickly, within a matter of 15 to 20 minutes. I think some of that has got to do with the fact that the longitudinal expansion can't happen. It can expand radially, but it can't really blow out longitudinally, so I think there may be some merit of the tether itself,” he said.
With regard to how patients were chosen for the study, Muller argued that there hadn’t been “selective screening of sick patients” and that all were “particularly high risk.”
However, when the study first began, about two-thirds of patients were excluded due to degenerative valve disease, small ventricles, and septal bulges, he said. With more experience using echocardiography to determine who would likely have a successful procedure, the number of patients excluded has come down.
The Italian Experience
In a separate presentation, Francesco Bedogni, MD (Policlinico San Donato, Milan, Italy), showed findings from GIOTTO, an observational, prospective Italian registry of 1,049 MitraClip (Abbott) procedures done at 22 centers between February 2016 and June 2018.
Baseline MR was grade 4 in almost 80% of patients and more than two-thirds of patients had functional MR. Overall, 81.8% of patients were classified as NYHA III or IV at baseline. Mean LVEF was 37%, and patients on average spent 16 days in the previous year hospitalized for heart failure.
Procedural success was achieved in 95.6% with more than half of patients receiving two or more MitraClips. After the procedure, more than half of patients had MR grade 1, and MR grade 3 or 4 was reported in less than 10%. Of the 3.6% of patients who reported complications, vascular complications, cardiac tamponade, partial detachment, and persistent severe MR were most common.
By 30 days, 32 patients died (3.1%), most often due to procedural complications (15.6%) or multiorgan failure or cardiogenic shock (92%).
“The GIOTTO registry is going to be the largest study available on MitraClip that is still enrolling,” Bedogni said. “Basic and demographic follow-up will help clarify the real impact of MitraClip in the management of mitral regurgitation and heart failure. Acute results are certainly promising. This procedure is safe and effective.”
Although Bedogni “seemed concerned to justify the importance of another registry for MitraClip,” Feldman said, “the therapy has changed a lot in the last decade, and I think having a very up-to-date snapshot of how the procedure works today is very useful.”
Interestingly, the information gathered regarding the number of clips implanted per patient seems to imply that “we are more confident about adding a second device, in some cases, and also more confident about stopping with one when we think that's the best we can do,” he added.
Bedogni added that these patients will be followed through 5 years to “try and understand the recurrence of MR, rehospitalization, mortality, [and] hard endpoints.”
COAPT, the large randomized controlled trial looking at MitraClip for functional MR is due later this year.
Mitral Valve Calcification
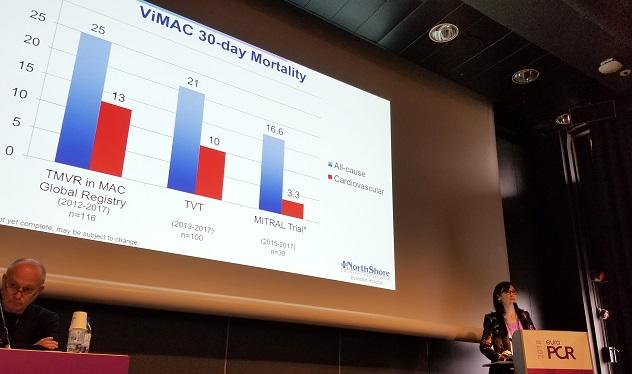
Next, Mayra Guerrero, MD (Evanston Hospital), who has previously presented positive feasibility findings from the MITRAL trial of patients who were screened to undergo TMVR with mitral annular calcification (MAC), showed new real-world data from the STS/ACC/TVT registry treated between 2013 and 2017.
Of the 100 patients, half received a Sapien 3 (Edwards Lifesciences) device, and 42% and 43%, respectively, were treated through transapical and transseptal access. Technical success at exit from the cath lab was 74%, and procedural success at 30 days was 48.6%. The most common causes for failure were need for a second valve (14%) and LVOT obstruction (10%).
Eighteen patients died in the hospital—10 for cardiovascular reasons—and another two died by day 30. There were no MIs or instances of valve thrombosis or endocarditis, but four patients had an ischemic stroke, seven needed new hemodialysis, and three needed a new pacemaker.
Echo analysis at 30 days showed 69.2% of patients with none/trace MR. Additionally, more than 60% of patients were classified as NYHA class I or II by 30 days.
TMVR in MAC “is a challenging procedure associated with complications and mortality,” Guerrero concluded. “There’s still work that needs to be done, but I think we are moving in the right direction. One of the key drivers of better outcomes has been what we have learned from CT analysis. . . . Further efforts are needed to improve overall outcomes and the long-term effect is not known and requires further evaluation.”
Following the presentation, panelist Olaf Wendler, MD, PhD (King’s College Hospital, London, England), asked whether these data will be able to lead to the creation of a “list of anatomical criteria to give people an idea [that] their patient may be more or less suitable in terms of placement and LVOT obstruction.”
Guerrero replied that she ultimately envisions this information being available via an app where physicians can plug in patient characteristics and know the likelihood of procedural success.
A Nonclip Device
Lastly, Simon Redwood, MD (St. Thomas’ Hospital, London, England), presented 1-year results from the MAVERIC trial of the transcatheter annular reduction therapy ARTO system (MVRx).
The ARTO device shortens the minor axis of the mitral valve through the implantation of an adjustable bridge anchored between the lateral wall and the septum. It is delivered venously, and can be sized to reshape the mitral annulus to achieve the desired reduction in MR.
As reported by TCTMD, 6-month results showed continued improvement in MR, as well as safety, among a cohort of 45 Australian and European patients. Findings at 1 year were maintained, with a total of eight deaths (five due to cardiovascular causes); no additional strokes or MIs occurred beyond 6 months. Five patients were hospitalized for heart failure (11.7%).
Overall, 92% of patients had an MR grade of 2+ or lower by 1 year, and 80% were considered NYHA class I or II. Additionally, the ARTO system reduced the AP annulus diameter by 15% from baseline, and LV end-systolic volume, LV end-diastolic volume, and left atrial volume were all reduced by 15% to 20%.
“It’s early days and it's only 45 patients, but I think we can conclude that the ARTO system does reduce the AP diameter of the mitral valve and it immediately and significantly reduces mitral regurgitation and it improves functional class and these improvements are maintained out to 1 year,” Redwood said. “This is the only nonclip transcatheter mitral valve device with 100% reporting echo and clinical follow-up at 1 year.”
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Muller DWM. CE Mark study of transcatheter mitral valve replacement for severe mitral regurgitation: 30-day outcomes for the first 100 patients. Presented at: EuroPCR 2018. May 23, 2018. Paris, France.
Bedogni F. GISE registry of transcatheter treatment of mitral valve regurgitation (GIOTTO): epidemiology and acute results. Presented at: EuroPCR 2018. May 23, 2018. Paris, France.
Guerrero M. 30-day outcomes of transcatheter mitral valve replacement in native mitral valve disease with severe mitral annular calcification in the United States: data from the STS/ACC/TVT registry. Presented at: EuroPCR 2018. May 23, 2018. Paris, France.
Redwood S. The mitral valve repair clinical (MAVERIC) EU/AU trial. Presented at: EuroPCR 2018. May 23, 2018. Paris, France.
Disclosures
- Muller reports receiving grant/research support from Tendyne and Medtronic and consulting fees/honoraria from Medtronic, Abbott, Boston Scientific, and Cephea.
- Bedogni reports receiving honoraria or consulting fees from Abbott, Boston Scientific, and Medtronic.
- The STS/ACC/TVT registry was supported by the American College of Cardiology Foundation’s National Cardiovascular Data Registry (NCDR) and the Society of Thoracic Surgeons National Database.
- Guerrero reports receiving research grant support from and proctoring for Edwards Lifesciences; serving as a consultant to and on the speaker’s bureau for Abbott and Boston Scientific; and receiving research grants from the ACC/STS NCDR.
- Redwood reports serving as a study proctor for MVRx.
- Feldman reports receiving institutional research grants and honoraria from Abbott, Boston Scientific, Edwards Lifesciences, and Gore.


Comments