TriCLASP: Tricuspid Repair With Pascal Safe, Effective at 30 days
With many devices in development to treat TR, Edwin Ho says having options will be key in tailoring treatment to anatomy.

PARIS, France—Thirty-day outcomes of the TriCLASP postmarket study of the Pascal and Pascal Ace transcatheter repair systems (Edwards Lifesciences) show promise for reducing tricuspid regurgitation (TR) and improving quality of life.
The results follow similar data reported after the device was cleared for commercial use in Europe—CE Mark approval in Europe for the treatment of mitral regurgitation was announced in February 2019 and the TR approval came in October 2020.
According to Stephan Baldus, MD (Kardiologie Uniklinik Köln, Germany), where the Pascal device shines above other options—both available and in development—is in the “maneuverability [and] steerability of the device inside the valve and inside the right ventricle.” Baldus presented the TriCLASP data today at EuroPCR 2022. “In a way, [that abolishes the] risk of being entangled given that you can elongate the system and safely retract it.” The second unique feature is that it can independently grasp tricuspid valve leaflets to increase the likelihood of proper positioning.
For the TriCLASP study, Baldus and colleagues enrolled 74 patients (mean age 80 years; 58% female) with primarily functional (84.2%) but also degenerative (5.3%) and mixed/other (10.5%) TR. TR was severe or greater in 83% of the population and 96% had atrial fibrillation.
All but two patients had successful device implantation (97%), and procedural and clinical success were each 78%. The mean number of devices implanted per patient was 1.8, and patients stayed in the hospital for an average of 5.1 days.
Among the 67 patients in whom 30-day follow-up data were available, the total composite “massive adverse event rate” was 3.0%. One patient died 14 days after the procedure after having a stroke following a massive bleeding event, and another died of pneumonia after 40 days. Three patients were hospitalized for heart failure.
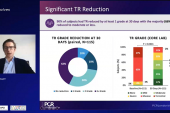
Core lab assessment showed marked improvement in TR severity, with more than 90% reporting no more than moderate TR after 30 days (P < 0.001); 88% achieved at least one TR grade reduction. Additionally, NYHA class improved, with 56% graded to be class I or II at 30 days compared with slightly more than 20% at baseline (P < 0.001). Kansas City Cardiomyopathy Questionnaire (KCCQ) score increased by about 13 points and 6-minute walk distance went up by about 38 meters (both P < 0.001).
Baldus said the study will be ongoing with follow-up through 5 years.
Commenting on the study findings, session co-moderator Corrado Tamburino, MD, PhD (University of Catania, Ferrarotto Hospital, Italy), said a similar scenario is taking place for TR as was seen for mitral regurgitation. “We implanted the clip for many years without any evidence from studies,” he said. “It was an unmet clinical need.”
Baldus agreed and said that even so, the randomized clinical trials ongoing for TR are still needed and will likely be “better” than they were in the mitral space since “we are [getting the data] at an earlier stage.”
Several Device Options
During the same session, Philipp Lurz, (Heart Center Leipzig, Germany), presented additional 30-day data from the TriClip bRIGHT study on the first 300 patients implanted with the TriClip and TriClip G4 edge-to-edge repair devices (Abbott). TCTMD previously covered 30-day data on the first 200 patients last year.
Implant success was 98% and acute procedural success was 91%; two clips were used per patient on average. At 30 days, 71% of patients reported moderate or lower TR (P < 0.0001). Among the 108 patients who received the newer TriClip G4 device, independent grasping was used in 69% of cases and 75% reported moderate or lower TR at 30 days (P < 0.0001).
Overall, three major adverse events (1.0%) were reported, including one CV death and one stroke. Four patients required tricuspid valve reintervention or reoperation (1.3%) and 20 reported major bleeding (6.7%). NYHA functional class improved, with 78% in class I or II at 3 days, and KCCQ score improved by 18 points (P < 0.0001 for both).
Lastly, Rebecca Hahn, MD (NewYork-Presbyterian/Columbia University Irving Medical Center, New York, NY), presented 1-year data from the CLASP TR study, previously covered by TCTMD at the American College of Cardiology 2022 Scientific Session last month.
“We're starting to build up a good body of evidence that's really telling us that these procedures are very feasible, [and] there's pretty good safety data as well, which I think is very important,” session co-moderator Edwin Ho, MD (Albert Einstein College of Medicine, Bronx, NY), told TCTMD. “It gives us the time and space to develop the even more robust clinical trials that we need from the clinical impact perspective, especially around prognosis. Those studies often take longer. So then to see the initial symptom benefit that we're seeing in these studies is really important.”
With many up-and-coming devices in the TR space, it’s good to have options, Ho continued. “The whole idea about tailoring device selection is one of the hot topics in the field in general,” he said. “It will be something that we have to customize to anatomy so it's exciting to see all of these options. In a lot of the sessions here, people have done slight predictions in how they think the field is going, and I think it's hard to tell, but the more we learn and also the timing of when we're treating patients, earlier versus later, we can wind up defining what we use more and more in the future.”
Right now, many patients with TR are presenting late in their disease progression, which makes them particularly difficult to treat. “As the evidence builds up in the future, I think that these patients will actually be recognized a little earlier, be treated potentially a little earlier, which helps avoid the extremes in anatomy,” Ho said. “That's why I think the field is a little bit of a shifting space, that over time I think we'll actually continue to evolve in a lot of ways.”
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Baldus S. 30-day outcomes for transcatheter tricuspid repair: TriCLASP post-market study. Presented at: EuroPCR 2022. May 19, 2022. Paris, France.
Hahn R. Transcatheter tricuspid valve repair: CLASP TR study one-year results. Presented at: EuroPCR 2022. May 19, 2022. Paris, France.
Lurz P. Real-world outcomes for tricuspid edge-to-edge repair: initial 30-day results from the TriClip bRIGHT study. Presented at: EuroPCR 2022. May 19, 2022. Paris, France.
Disclosures
- Baldus reports receiving lecture fees/honoraria from Edwards Lifesciences and Abbott and research grants from Abbott.
- Hahn reports receiving speaker fees from Abbott Structural, Baylis Medical, Edwards Lifesciences, and Philips Healthcare; having institutional consulting contracts with Abbott Structural, Boston Scientific, Edwards Lifesciences, Medtronic, and Novartis; having stock options with Navigate; and serving as the Chief Scientific Officer for the echocardiographic core laboratory at the Cardiovascular Research Foundation.
- The TriClip bRIGHT study is sponsored by Abbott.
- Lurz reports serving as a consultant to Abbott, Edwards Lifesciences, and Medtronic.
- Ho reports receiving honoraria from GE Healthcare.



Comments