Valve-in-Valve TAVR: Mortality, Adverse Events Similar to Redo Surgery at 30 Days
Longer-term and potentially randomized data, especially in low-risk patient cohorts, are needed in order to expand this procedure, one expert says.
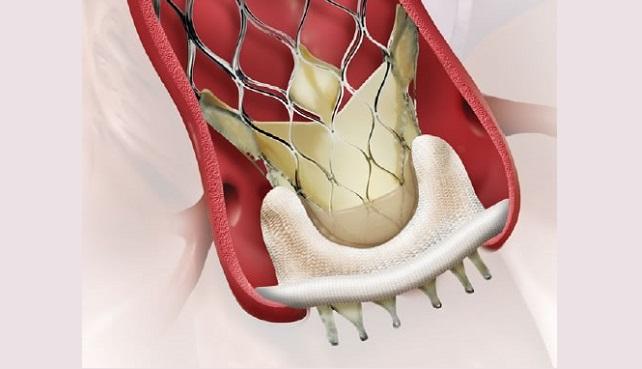
Among patients with failing bioprosthetic aortic valves, transcatheter valve-in-valve (ViV) and standard secondary surgical aortic valve replacement are each associated with similar rates of mortality and adverse events at 30 days, according to a new meta-analysis.
“With an equivalent short-term performance, ViV-TAVI offers an acceptable alternative for the treatment of bioprosthetic valve dysfunction especially in [a] high-risk patient who might be denied repeat surgery—the current standard of care,” write lead author Saroj Neupane, MD (St. John Hospital and Medical Center, Detroit, MI), and colleagues.
For the study, published online last week ahead of print in the American Journal of Cardiology, the researchers included 489 patients with bioprosthetic valve dysfunction from four observational studies who were treated with either ViV TAVR (n = 227) or repeat surgery (n = 262). Those in the TAVR arm were older with a higher prevalence of comorbidities, and hence had higher EuroSCORE values.
There were no differences between the TAVR and surgery arms in terms of 30-day mortality (5% vs 4%; OR 1.08; 95% CI 0.44-2.62), stroke (2% vs 2%; OR 1.00; 95% CI 0.28-3.59), MI (2% vs 1%; OR 1.08; 95% CI 0.27-4.33), and acute kidney injury requiring dialysis (7% vs 10%; OR 0.80; 95% CI 0.36-0.17) at 30 days, although the rate of permanent pacemaker implantation was lower in the TAVR arm (9% vs 15%; OR 0.44; 95% CI 0.24-0.81).
Patients who underwent ViV TAVR had a higher incidence of residual mild or greater aortic regurgitation than those who had redo surgery in the three studies with these data. The residual mean gradient was higher in the ViV TAVR than surgery patients in each of these studies as well.
Commenting on the study for TCTMD, Tarun Chakravarty, MD (Cedars-Sinai Heart Institute, Los Angeles, CA), said it “gives confidence that the procedure is technically feasible and does not lead to any worse outcomes compared to redo surgery. So that's very reassuring.”
If anything surprised him about the results, it was the low rate of mortality following surgery, not necessarily ViV TAVR. However, this could be explained by selection bias, in that the TAVR-treated patients were at higher risk, he said. “These are not comparable populations.”
Still, “it’s reassuring that stroke rates are no higher with valve-in-valve [TAVR],” said Chakravarty. “For high-risk patients who are not candidates for redo surgery, I think this study again proves the point that valve-in-valve is a very reasonable option. But for low-risk patients, I think it’s important to look at the long-term outcomes.”
Also, that this study shows higher gradients with ViV TAVR compared with surgery is “a little concerning, because the higher gradients have been known to translate into worse outcomes in the long-term and this study only reports 30-day data,” he observed.
Chakravarty suggested that future trials follow especially low-risk patients out to at least 5 years, as the 30-day window only speaks to procedural outcomes. The next study would “ideally be a randomized trial of redo surgery versus valve-in-valve in low-risk patients,” he said. “But such a trial may not be feasible. In which case, at least a well-designed registry that's evaluating all outcomes of valve-in-valve [should happen].”
Photo Credit: Medtronic.
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Neupane S, Singh H, Lämmer J, et al. Metanalysis of transcatheter valve-in-valve implantation versus redo aortic valve surgery for bioprosthetic aortic valve dysfunction. Am J Cardiol. 2018;Epub ahead of print.
Disclosures
- Neupane and Chakravarty report no relevant conflicts of interest.


Comments