Live From Washington: ACC 2017, Day Two
This blog aims to capture headline news and random tidbits on the ground at ACC 2017. It will be updated throughout the day.

WASHINGTON, DC— The big news to rock ACC 2017 today was a safety alert from the US Food and Drug Administration (FDA) about a risk of major adverse cardiac events seen in ABSORB III at 2 years. That alert appeared in my email inbox 47 minutes before the embargo was to lift on these data for journalists covering the meeting, and arrived an hour and 47 minutes before the data were slated for presentation in the ACC Main Tent.
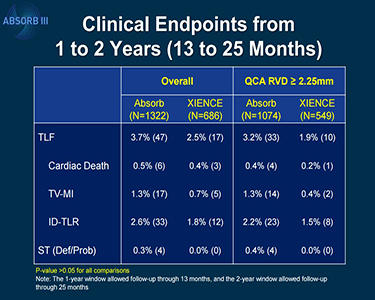 The FDA letter notes that using the Absorb BVS (Abbott Vascular) increases the risk of target lesion failure—a composite of cardiac death, target-vessel MI, and ischemia-driven TLR—when compared with patients who received the everolimus-eluting metallic stent (Xience; Abbott Vascular). In an analysis of 2-year data from ABSORB III, the rate of target lesion failure was 11.0% in patients who received Absorb and 7.9% in patients who received Xience (P = 0.03).
The FDA letter notes that using the Absorb BVS (Abbott Vascular) increases the risk of target lesion failure—a composite of cardiac death, target-vessel MI, and ischemia-driven TLR—when compared with patients who received the everolimus-eluting metallic stent (Xience; Abbott Vascular). In an analysis of 2-year data from ABSORB III, the rate of target lesion failure was 11.0% in patients who received Absorb and 7.9% in patients who received Xience (P = 0.03).
More details emerged in the LBCT session itself (read reporter Michael O'Riordan's story here.)
The reaction on Twitter was swift, with some observers echoing the comments made by some of the LBCT panelists, stressing the need for proper implantation techniques. Others, however, asked whether this device should remain on the market, given the safety signal.
But not for all patients. It is highlighting to use it in the right patients. https://t.co/t5188UmdA1
— J.P. Reilly (@JPReillyMD) March 18, 2017
@ShelleyWood2 hard times for #ABSORB! #ACC17
— R Ladeiras Lopes (@rladeiraslopes) March 18, 2017
@ShelleyWood2 @TCTMD I think we (the physicians) have been already warned by our own clinical practice (&common sense) #ACC17
— XFR (@xacobeflores) March 18, 2017
Ellis, speaking to the press, pointed out that, in his opinion, the ABSORB III 2-year presented today are reassuring in comparison with the ABSORB II and ABSORB Japan data. “If anything, this calms the water.” Asked whether it might be worth pausing use of the device until there are more years of data to show that, long-term, this disappearing device delivers on its promise, Ellis said no. “I think the concerns are legitimate, but I think we have enough data to see that this is not a dangerous device and we need more data. And we’re not going to get more data unless we use it.”
Also presented in LBCT III were the DEFINE-FLAIR and iFR-SWEDEHEART trials, which reporter Caitlin Cox covered in depth here. In both studies, instantaneous wave-free ratio (iFR), the newer technology, was associated with a noninferior risk of MACE at 1 year compared with fractional flow reserve (FFR). FFR involves using adenosine, as well as measurement of the pressure gradient across a lesion during hyperemia, whereas iFR is calculated during diastole and does not require use of hyperemic agents.
Commenting for Caitlin’s story, however, William F. Fearon, MD (Stanford University Medical Center, Stanford, CA), said, “It’ll be interesting to see how [this] all unfolds, whether it really changes people’s behavior. I’m probably biased because I do FFR all the time, but I don’t find that adenosine is the big hindrance.” Side effects typically amount to “a minute or two of heaviness in the chest, shortness of breath. But if you warn the patient, in my experience they tolerate it fine,” he observed.
For more from day 2, check out Todd Neale's coverage of Compare-Acute, showing that using fractional flow reserve (FFR) to guide revascularization of all functionally significant lesions in the setting of acute STEMI appears to improve outcomes over treating only the culprit artery.Then there was DECISION-CTO, looking at revascularization versus optimal medical therapy in stable CAD patients with chronic total occlusions. Read reporter Yael Maxwell's story here.
* * *
The opening late-breaking clinical trial session on day 2 of the ACC 2017 was a mix of trials looking at ways of lowering things you don’t want and boosting the things you do, plus one aimed at not getting something that you never wanted in the first place, from a drug you may not be able to afford.
 EINSTEIN CHOICE, covered by TCTMD’s L.A. McKeown, looked at over 3,300 patients at risk of recurrent venous thromboembolism (VTE) randomized to 10 mg or 20 mg of rivaroxaban, or 100 mg of aspirin for up to 12 months. The trial found that major bleeding was similar between the 10- and 20-mg rivaroxaban groups and aspirin, but both rivaroxaban doses had lower rates of recurrent VTE—the study’s primary endpoint—compared with aspirin. Full results from EINSTEIN CHOICE are here.
EINSTEIN CHOICE, covered by TCTMD’s L.A. McKeown, looked at over 3,300 patients at risk of recurrent venous thromboembolism (VTE) randomized to 10 mg or 20 mg of rivaroxaban, or 100 mg of aspirin for up to 12 months. The trial found that major bleeding was similar between the 10- and 20-mg rivaroxaban groups and aspirin, but both rivaroxaban doses had lower rates of recurrent VTE—the study’s primary endpoint—compared with aspirin. Full results from EINSTEIN CHOICE are here.
GEMINI-ACS-1 was also a rivaroxaban trial, this time on top of P2Y12 inhibition against standard dual antiplatelet therapy in the setting of acute coronary syndromes.
 As Todd Neale reports, the rate of TIMI clinically significant bleeding not related to CABG in this 3,000-plus patient trial was roughly similar in both groups. The results, on top of previous trials including WOEST and PIONEER-AF-PCI, suggest that the increased bleeding seen in patients receiving triple therapy is “strongly affected” by aspirin, investigators said. GEMINI-ACS-1 was not, however, powered to look at ischemic endpoints, warranting a larger randomized trial looking at net clinical benefits, they added.
As Todd Neale reports, the rate of TIMI clinically significant bleeding not related to CABG in this 3,000-plus patient trial was roughly similar in both groups. The results, on top of previous trials including WOEST and PIONEER-AF-PCI, suggest that the increased bleeding seen in patients receiving triple therapy is “strongly affected” by aspirin, investigators said. GEMINI-ACS-1 was not, however, powered to look at ischemic endpoints, warranting a larger randomized trial looking at net clinical benefits, they added.
GEMINI-ACS ischemic endpoints also promising but remember, it is UNDERPOWERED #ACC17 pic.twitter.com/szZtR0f7zu
— JKH MD (@netta_doc) March 18, 2017
Read details of the trial here.
 CARAT, like other trials that have tried and failed to boost HDL cholesterol, with clinical benefit, appears to be fool’s gold. The trial tested CER-001, an injectable synthetic HDL-C used in secondary prevention, but found no effect on coronary plaque progression as measured by plaque volume on IVUS. “The lack of benefit with short-term therapy with CER-001 3 mg/kg in CARAT suggests that this is not a promising strategy for ACS patients,” presenter Stephen Nicholls, MD (Medical Research Institute, Adelaide, Australia), observed. “With each disappointing study result, the considerable challenge remains.”
CARAT, like other trials that have tried and failed to boost HDL cholesterol, with clinical benefit, appears to be fool’s gold. The trial tested CER-001, an injectable synthetic HDL-C used in secondary prevention, but found no effect on coronary plaque progression as measured by plaque volume on IVUS. “The lack of benefit with short-term therapy with CER-001 3 mg/kg in CARAT suggests that this is not a promising strategy for ACS patients,” presenter Stephen Nicholls, MD (Medical Research Institute, Adelaide, Australia), observed. “With each disappointing study result, the considerable challenge remains.”
EBBINGHAUS, the cognitive substudy from yesterday’s FOURIER trial—the large CVD outcomes trial for the PCSK9 inhibitor, evolocumab—closed down the LBCT 2 session, showing no evidence of a difference in “spatial working memory strategy index of executive function” between the evolocumab and usual-care groups.
There were also no differences in everyday cognition out to 2 years, with no differences according to the degree of LDL-C lowering. Full EBBINGHAUS results will be on the site soon.
EBBINGHAUS results are in from #FOURIER - no cognitive difference was found between Evolocumab vs placebo even at very low LDL-C. #ACC17 pic.twitter.com/tvp9GWWJTv
— Erin D. Michos (@ErinMichos) March 18, 2017
Shelley Wood was the Editor-in-Chief of TCTMD and the Editorial Director at the Cardiovascular Research Foundation (CRF) from October 2015…
Read Full Bio

Comments