CRISPR/Cas-9 Gene Editing Shows Promise in Transthyretin Amyloidosis
As the first CRISPR trial to show “unequivocal success,” Kiran Musunuru calls it a “milestone for modern medicine.”
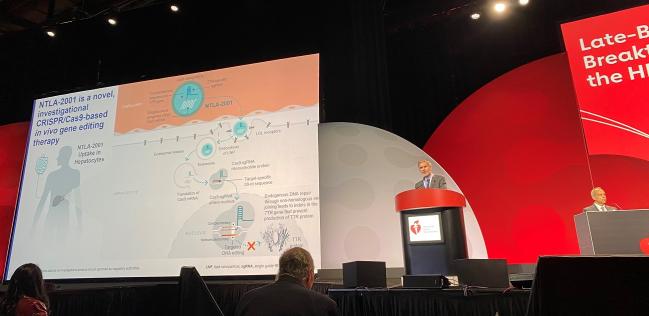
CHICAGO, IL—A novel CRISPR/Cas-9-based gene-editing technique seems to safely diminish the production of a protein linked to worsening outcomes in patients with transthyretin (ATTR) amyloidosis cardiomyopathy, according to first-in-human data.
A progressive and fatal disease, ATTR can be wild type or hereditary affecting 200,000-500,000 and 50,000 patients worldwide, respectively. Current treatment options are limited and include expensive drugs like tafamidis and tafamidis meglumine (Vyndamax and Vyndaqel; Pfizer), patisiran (Onpattro; Alnylam), and inotersen (Tegsedi; Akcea).
“These data are consistent with previously reported data from the polyneuropathy arm of the trial and further support and extend early findings that demonstrate the promise of CRISPR Cas-9 based in vivo gene editing in humans,” said Julian D. Gillmore, MD, PhD (Royal Free Hospital, London, England), who presented the results here today during a late-breaking trial session at the American Heart Association (AHA) 2022 Scientific Sessions.
Discussing the trial during a press conference, Kiran Musunuru, MD, PhD (University of Pennsylvania, Philadelphia), said the study is important because “it's part of the first wave of trials putting CRISPR into the body to treat a variety of diseases, including a genetic form of blindness, familial hypercholesterolemia, hereditary angioedema, HIV, Duchenne muscular dystrophy. TTR gene editing stands out because it’s the very first CRISPR trial to show unequivocal success. You see that in the greater than 90% reduction of TTR in the blood. So, in my view, that makes it a milestone for modern medicine.”
First-in-Human Data
For the study, Gillmore and colleagues tested a novel CRISPR/Cas-9-based in vivo gene-editing therapy called NTLA-2001 in 12 male patients (median age 75 years; 83% wild type). The compound is delivered through a single IV infusion and binds with the LDL receptors of the patient’s hepatocytes to target their DNA, disabling the TTR gene and hence the production of TTR protein, which has previously been shown to have benefit for ATTR patients. Three dose groups were studied here, including:
- 1.0 mg/kg for three NYHA class I/II patients
- 0.7 mg/kg for six NYHA III patients
- 0.7 mg/kg for three NYHA I/II patients
All patients received the full dose of the therapy. The treatment was well tolerated across the spectrum of patients with 67% reporting only mild or moderate adverse events, including infusion-related reactions in two patients, one of which was considered grade 3 and resolved without further issue. There were also no clinically significant lab findings beyond transient grade 1 liver enzyme elevations in some.
By day 28, all patients achieved TTR reductions of at least 90%. Specifically, those in the NYHA class III 0.7 mg/kg and NYHA class I/II 1.0 mg/kg cohorts achieved 94% and 92% TTR reductions, respectively, at 4 months. The NYHA class I/II 0.7 mg/kg group had achieved 93% TTR reductions at 6 months.
Comparing the study findings to those of APOLLO-B for the small-interfering RNA (siRNA) therapy patisiran presented earlier this year, Musunuru emphasized that patients receiving patisiran “have to get an IV infusion that lasts more than an hour in the infusion clinic every 3 weeks, chronically, for the rest of their lives. In contrast, gene editing is a one and done proposition. You receive a single treatment, you turn off the TTR gene permanently, and the effects are durable and likely lasts for lifetime.”
APOLLO-B reported a mean 87% reduction in TTR, he continued, “so the effect of gene editing matches and actually exceeds the effect of patisiran.” Musunuru added that while patisiran led to “substantially better functional capacity and quality of life compared to placebo,” the results of the CRISPR/Cas9 study indicate that “future clinical trials for gene editing [will] have the same beneficial effects for patients and possibly a mortality benefit as well.”
As for cost, Gillmore told TCTMD it is currently too early to comment on how the price for gene editing might compare to currently available treatment options.
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Gilmore JD. First-in-human in vivo CRISPR/Cas9 editing of the TTR gene by NTLA-2001 in patients with transthyretin (ATTR) amyloidosis with cardiomyopathy. Presented at: AHA 2022. November 5, 2022. Chicago, IL.
Disclosures
- Gillmore reports receiving consultancy fees from Alnylam, Ionis, AstraZeneca, Pfizer, Intellia, ATTRalus and NovoNordisk and receiving grant support from Alnylam Pharmaceuticals.




Comments