Dapagliflozin Safe, Effective in Diabetes Regardless of Background CV Meds
The findings should reassure physicians that SGLT2 inhibitors have a role to play, even in the setting of other cardiorenal drugs.

Dapagliflozin improves a range of cardiovascular and renal outcomes in patients with type 2 diabetes without interacting adversely with other guideline-recommended medications, an analysis of DECLARE-TIMI 58 confirms.
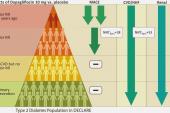
Most clinicians working in this space have had confidence in the safety and efficacy of the sodium-glucose cotransporter 2 (SGLT2) inhibitors, based on the fact that patients in the trials have typically been taking one or more background therapies. And in heart failure (HF) specifically, US and European guidelines recommend initiation and titration of all major drug classes as swiftly as possible as part of the so-called “four-pillar” approach. This new analysis in diabetic patients, who often have cardiovascular and renal comorbidities, was specifically focused on the safety of dapagliflozin (Farxiga; AstraZeneca) with and without background use of other drugs—some of which also have antihypertensive and glucose-lowering effects.
The paper should help shore up confidence for prescribing the SGLT2 inhibitor in a variety of settings, senior author Stephen D. Wiviott, MD (TIMI Study Group, Boston, MA), told TCTMD.
“That was the concept behind this analysis,” he said. “We hope that it will provide some comfort in use of these agents with other CV medications, particularly diuretics in stable patients like those in DECLARE.”
The study, with lead author Kazuma Oyama, MD, MPH (Tohoku University Graduate School of Medicine, Sendai, Japan), was published last week in JAMA Cardiology.
“We believe that our findings will help physicians to initiate or maintain SGLT2 inhibitors, the ‘statins of the 21st century,’ without any concern about concurrent use of CV medications, in a wide range of patients who have its indication,” Oyama told TCTMD in an email.
Reassurances
As previously reported by TCTMD, DECLARE-TIMI 58, with over 17,000 patients, was one of the early, large trials to establish a role for an SGLT2 inhibitor in reducing the risk of CV death or HF hospitalization in patients with type 2 diabetes. The current, prespecified analysis zeroed in on the 81% of patients also taking ACE inhibitors or ARBs, the 53% using beta-blockers, the 36% using diuretics, and the 4% using mineralocorticoid receptor antagonists (MRAs) at baseline.
Analyzed for each background medication, changes in blood pressure and kidney function between dapagliflozin and placebo were similar at 48 months, regardless of concurrent therapies, individually, or for patients receiving the three most common drug classes together. Moreover, dapagliflozin consistently reduced rates of cardiovascular death/HF hospitalizations, as well as HF hospitalizations alone, regardless of background meds.
There were no signs of worrisome volume depletion, acute kidney injury, or hyperkalemia in patients randomized to SGLT2 inhibition as compared with placebo. Of note, the beneficial effect of dapagliflozin on the kidney-specific composite outcome appeared to be greater in the subgroup of patients not already taking diuretics (P = 0.003 for interaction). This could be a chance finding, the authors say, or might point to a kidney-protective effect of SGLT2 inhibitors.
“These data support efforts to develop and implement strategies to optimize the use of SGLT2 inhibitors in a broad range of patients with type 2 diabetes regardless of background therapy,” Oyama et al conclude.
More to Come
Because DECLARE-TIMI 58 was not an HF trial, very few patients were taking MRAs and none were on sacubitril/valsartan (Entresto; Novartis), making it impossible to draw conclusions about performance of the SGLT2 inhibitor on top of angiotensin receptor-neprilysin inhibitors (ARNIs).
“Because of the timing of approval of Entresto and the conduct of the DECLARE trial, there was an insufficient number of patients on an ARNI to be able to address the use of these agents together,” Wiviott said. “We do anticipate a cohort of patients in DAPA ACT HF on ARNIs, which will allow us to address the coadministration of these with an SGLT2 inhibitor.”
DAPA ACT HF-TIMI 68 is current enrolling, he noted, and is comparing dapagliflozin with placebo in patients with acute decompensated heart failure. “We hope [it] will be the definitive trial to assess the safety and efficacy of SGLT2 inhibition in hospitalized patients who are receiving multiple and often new cardiac medications at the same time as the SGLT2 inhibitors,” he said.
Shelley Wood was the Editor-in-Chief of TCTMD and the Editorial Director at the Cardiovascular Research Foundation (CRF) from October 2015…
Read Full BioSources
Oyama K, Raz I, Cahn A, et al. Efficacy and safety of dapagliflozin according to background use of cardiovascular medications in patients with type 2 diabetes: a prespecified secondary analysis of a randomized clinical trial. JAMA Cardiol. 2022;Epub ahead of print.
Disclosures
- Oyama reports grants from the Uehara Memorial Foundation and JSPS Overseas Research Fellowships.
- Wiviott reports grants from AstraZeneca, Bristol Myers Squibb, Sanofi Aventis, and Amgen; grants and personal fees from Arena, Daiichi Sankyo, Eisai, Eli Lilly, and Janssen; grants and consulting fees from Merck; personal fees from Aegerion, Allergan, AngelMed, Boehringer Ingelheim, Boston Clinical Research Institute, Icon Clinical, Lexicon, Novo Nordisk, Pfizer, St. Jude Medical, Xoma, Servier, AstraZeneca, and Bristol Myers Squibb; his spouse being employed by Merck; and, as a member of the TIMI Study Group, institutional research grant support through Brigham and Women’s Hospital from Abbott, Amgen, Anthos Therapeutics, ARCA Biopharma, AstraZeneca, Bayer HealthCare Pharmaceuticals, Daiichi Sankyo, Eisai, Intarcia, Ionis Pharmaceuticals, Janssen Research and Development, MedImmune, Merck, Novartis, Pfizer, Quark Pharmaceuticals, Regeneron Pharmaceuticals, Roche, Siemens Healthcare Diagnostics, Softcell Medical, The Medicines Company, and Zora Biosciences.




Comments