Hints of Benefit With Catheter-Directed Thrombolysis in Intermediate/High-Risk PE
Any potential positive effects seen in CANARY, cut short by the COVID-19 pandemic, have to be confirmed in future trials.
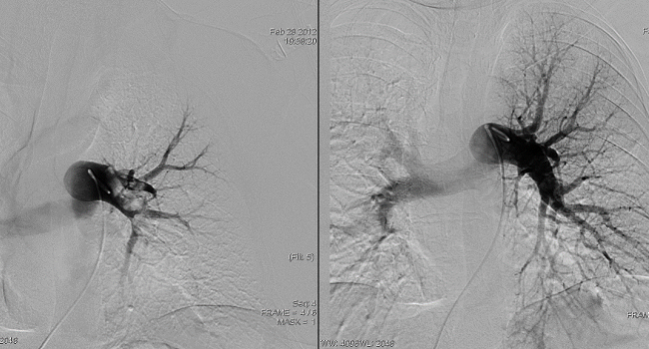
Photo Credit: Adapted from Ruzsa, Z. Catheter directed thrombolysis and mechanical thrombectomy pulmonary embolism. Presented at: TCT 2014. Washington, DC.
Conventional catheter-directed thrombolysis (CDT) may provide clinical benefits for patients with acute intermediate-to-high-risk pulmonary embolism (PE) when compared with anticoagulation alone, although the results, from the prematurely terminated and underpowered CANARY trial, need to be confirmed in future studies.
Patients treated with CDT were less likely to have an RV/LV ratio greater than 0.9, an echocardiographic measure that has been associated with adverse outcomes, at 3 months, but the difference in that primary endpoint did not reach statistical significance (4.3% vs 12.8%; P = 0.24), Parham Sadeghipour, MD (Iran University of Medical Sciences, Tehran), and colleagues report in a study published online today in JAMA Cardiology.
Some secondary outcomes, including the RV/LV ratio at 72 hours and the 3-month rate of death or RV/LV ratio > 0.9, favored conventional CDT over anticoagulation alone. The rate of major bleeding was low, with just one case of nonfatal major GI bleeding that occurred in the CDT group.
Senior author Behnood Bikdeli, MD (Brigham and Women’s Hospital, Boston, MA), speculated that if the trial had been able to proceed as planned instead of being halted at the outset of the COVID-19 pandemic in Iran, there would have been a significant benefit of CDT observed for the primary endpoint.
“Looking at all the secondary outcomes and the direction of results in the primary outcome, I think it’s safe to hypothesize the results would have been promising, but we wouldn’t know for sure,” he told TCTMD.
Despite the early stoppage, the trial is still the largest completed RCT of catheter-based interventions for patients with intermediate-to-high-risk PE, and when the results are pooled with other trials of CDT, rates of fatal, intracranial, and major bleeding remain low. “It’s unlikely that these catheter-based therapies are having a similar risk of major bleeding and intracranial bleeding compared with systemic lytics, and hopefully it’s a lot less,” Bikdeli said. “That’s reassuring, giving hope for the additional studies.”
The CANARY Trial
Bikdeli said that for the sickest patients with PE, those with high-risk (massive) PE, advanced treatments like systemic fibrinolysis, catheter-based therapies, and sometimes surgery are indicated. But there is less certainty when it comes to patients with intermediate-to-high-risk PE. In the PEITHO trial, conducted in that subset, full-dose systemic fibrinolytic therapy lowered the risk of clinical deterioration but at the cost of much more hemorrhagic stroke and extracranial bleeding, dissuading clinicians and guideline writers from recommending that approach.
Catheter-based interventions have been studied as a way to both deliver thrombolytic therapy directly to the pulmonary arteries and lower the necessary dose, possibly lessening bleeding risks. But most data come from observational or small randomized studies, and the clinical impact in comparison to standard-of-care anticoagulation has remained unclear.
That led investigators to design the CANARY trial, an open-label RCT conducted at two large cardiovascular centers in Tehran. Researchers enrolled patients with acute intermediate-to-high-risk PE who presented within 14 days of symptom onset and randomized them to conventional CDT plus heparin or anticoagulation monotherapy. CDT involved delivery of alteplase 0.5 mg/catheter/hour using Cragg-McNamara valved infusion catheters (Medtronic). Anticoagulation started with twice-daily subcutaneous enoxaparin, although patients in both groups could be transitioned to oral agents based on the discretion of the treating physicians.
The trial had a planned sample size of 288, but it had to be stopped early in February 2020 after 94 patients (mean age 58.4; 29% women) were enrolled; 85 eventually completed the 3-month echocardiographic follow-up.
The sooner we get definitive answers, the better we can serve our patients. Behnood Bikdeli
The primary outcome was an RV/LV ratio > 0.9, indicating an enlarged right ventricle, at 3 months, and that was observed in just two patients in the CDT arm and five in the anticoagulation monotherapy arm. The median RV/LV ratio at 3 months was lower following CDT (0.7 vs 0.8; P = 0.01).
An RV/LV ratio > 0.9 at 72 hours, a secondary outcome, was significantly less common in the CDT group (27.0% vs 52.1%; P = 0.01), as were the composite of all-cause death or an elevated RV/LV ratio (4.3% vs 17.3%; P = 0.048) and the rate of unrecovered RV function (6.2% vs 28.2%; P = 0.009) at 3 months. Three patients, all in the anticoagulation arm, died within 3 months; two deaths were considered to be related to PE and the third was possibly related to either PE or cancer.
The main safety outcome was BARC type 3 or 5 bleeding, and only one of these events occurred, a nonfatal major GI bleeding in the CDT group. There were no fatal or intracranial bleeds during the trial.
Where Do We Go From Here?
Most prior trials of CDT that have found improvements in short-term imaging endpoints have used catheters with ultrasound assistance. CANARY used a conventional infusion catheter because of the higher cost and limited availability of ultrasound catheters in the two study centers. The authors note that in the SUNSET sPE trial, ultrasound-assisted thrombolysis was no better than conventional CDT in patients with submassive PE, adding, however, that further studies are needed to compare the two modalities.
There are some ongoing or planned trials evaluating treatments for patients with intermediate-to-high-risk PE, including the HI-PEITHO and PE-TRACT trials of CDT, the PEITHO-3 trial of reduced-dose systemic fibrinolytic therapy, and studies of mechanical thrombectomy.
CANARY, Bikdeli said, “gives additional hope and more encouraging results with respect to safety [of CDT], making the case to enthusiastically follow the results of these recently initiated larger randomized trials of catheter-based therapies.”
For the time being, anticoagulation remains the go-to treatment for patients with intermediate-to-high-risk PE, he said, but added that signs of decompensation can spur use of more-advanced therapies. “Even if they have some further deterioration in their hemodynamic parameters or worsening oxygenation or have new syncope, I think that would be enough to consider advanced therapies,” Bikdeli explained, encouraging his colleagues to randomize patients into ongoing trials. “Because the sooner we get definitive answers, the better we can serve our patients.”
Commenting for TCTMD, Vikas Aggarwal, MBBS (University of Michigan, Ann Arbor), said the signals of benefit observed in CANARY are consistent with the prior studies in this space, adding that the difference in the primary endpoint didn’t reach statistical significance because the trial was underpowered after being stopped early.
Bleeding complications were rare, “which tells you that overall, essentially, either strategy of anticoagulation alone or catheter-directed lysis is safe in these patients,” Aggarwal said. “We have to wait for trials like HI-PEITHO and PE-TRACT that are starting to enroll now to get a definitive answer on the efficacy, but still, having a study that looks at outcomes not just at 72 hours but also at 3 months, it only adds to the evidence base that’s slowly building up on this topic.”
Larger studies are needed to definitively show whether a difference in the occurrence of an RV/LV ratio < 0.9 will translate into a meaningful impact on clinical outcomes, although it’s expected that it will, he said.
Importantly, Aggarwal noted, CANARY suggests that conventional CDT can provide results similar to those obtained with ultrasound-assisted technology, which has economic implications. “From a cost standpoint, there’s a big difference, because the ultrasound-assisted infusion catheter is severalfold more expensive compared to the non-ultrasound one,” he said.
Regardless of the specific technology used, it will be critical to identify higher-risk cohorts within the larger group of patients with intermediate-to-high-risk PE “to really be able to move the needle in terms of making a difference in actual clinical endpoints,” Aggarwal said. CANARY and other studies “do reassure us that the overall incidence of safety endpoints with catheter-directed lysis is not too high. Efficacy is still unproven.”
In an invited commentary, Elaine Hylek, MD (Boston University School of Medicine), points to the need for further research, too. “Given the heightened risk of mortality among patients with submassive pulmonary embolism, well-designed trials are needed to guide clinical practice in patient selection, optimal dose, timing of intervention, and effective mode of delivery,” she writes. “As highlighted by the authors, several of these are currently underway. More-discriminating risk models to better identify those patients at highest risk of decompensation would enable an earlier aggressive approach that would translate hopefully into more lives saved.”
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Sadeghipour P, Jenab Y, Moosavi J, et al. Catheter-directed thrombolysis vs anticoagulation in patients with acute intermediate-high-risk pulmonary embolism: the CANARY randomized clinical trial. JAMA Cardiol. 2022;Epub ahead of print.
Hylek EM. Catheter-directed treatment of submassive pulmonary embolism—a cautious step closer? JAMA Cardiol. 2022;Epub ahead of print.
Disclosures
- The study was supported by grants from Rajaie Cardiovascular Medical and Research Center and the Tehran Heart Center.
- Bikdeli reports receiving research support from the Mary Horrigan Connors Center for Women’s Health and Gender Biology at Brigham and Women’s Hospital, a Career Development Award by the American Heart Association, and VIVA Physicians; and being a consulting expert, on behalf of the plaintiff, for litigation related to two specific brand models of inferior vena cava filters.
- Aggarwal and Sadeghipour report no relevant conflicts of interest.
- Hylek reports serving on steering committees for Abbott, Bristol Myers Squibb, Janssen, and Medtronic; consulting for Bayer; and presenting at symposiums for Boehringer Ingelheim and Pfizer outside the submitted work.




Comments