New CT Guidelines for TAVR Emphasize Standardization, 4-D Acquisition, and Postprocedural Imaging
CT imaging, now known as the “gold standard” for structural procedural planning, is vital for patient selection, valve sizing, and risk assessment.
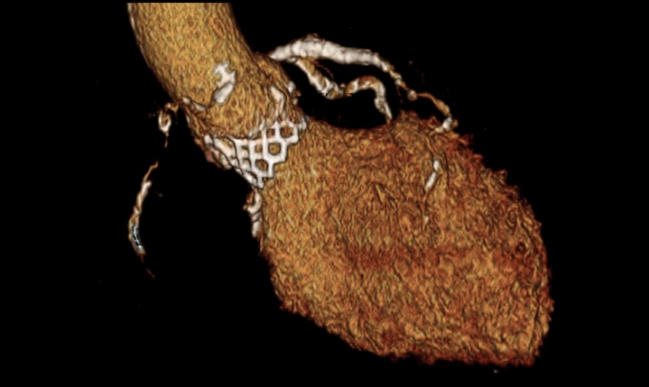
An updated set of guidelines for the use of computed tomography (CT) imaging for TAVR reflects the growing acceptance of the technology by operators in recent years, while also advocating for better standardization of techniques and acquisition.
The last set of guidelines, released by the Society for Cardiovascular Computed Tomography (SCCT) in 2012, were all about persuading physicians that a CT-based strategy was optimal for preprocedural TAVR planning, Philipp Blanke, MD (St Paul's Hospital & University of British Columbia, Vancouver, Canada), who served as the lead author for the new guidelines, told TCTMD.
“We don't have to convince people anymore,” he said. “It's established. Now [we’re] more focused on standardization of nomenclature, grading systems, and measurement techniques.”
SCCT’s latest document, published online last week ahead of print in the Journal of Cardiovascular Computed Tomography, is largely technical and provides specific guidance for various CT scanners and clinical scenarios, Blanke said. It was needed because the field of TAVR, and CT’s role within it, has changed immensely in the last few years, Blanke and colleagues write. “While CT was initially used primarily for the assessment of peripheral access, the role of CT has grown substantially and CT is now the gold standard tool for annular sizing, determination of risk of annular injury and coronary occlusion, and to provide coplanar fluoroscopic angle prediction in advance of the procedure.”
As the TAVR population grows, standardization becomes more important for gauging eligibility as well as valve sizing and identifying patients at high risk for adverse events like aortic root injury, Blanke said. “If you at the end of the day select the valve size based on one quantitative measurement, you have to make sure that this measurement is taken in a correct manner on a good quality CT and that the quantitative variable is then compared to a sizing chart which is provided by vendors, . . . so it's important that it's standardized.”
Because of how operators have come to rely upon this technology and the growing body of evidence to support it, Blanke said the new guidelines also place heavy “emphasis on the robustness of the acquisition.” In 2012, he explained, many operators only captured a selection of the cardiac cycle with CT. “Over the years we've learned that it's important to really have a good data set covering the entire cardiac cycle to determine the maximum aortic root dimensions, which are typically at one point in systole,” he said, noting that the paper gives specific recommendations on 4-D acquisition modes for various scanner systems.
At this point, Blanke said, “there are actually not that many questions left” regarding optimal use of CT in TAVR planning. “Of course, we will always see some adaptation with some new device iterations. Every device is different, and we have certain unique sizing algorithms. The underlying measurement technique will remain the same no matter which device you take,” he observed.
However, much remains unknown about using CT for follow-up imaging. Several research groups have published papers on subclinical leaflet thrombosis after TAVR in the past few years, yet Blanke acknowledged that there is not enough evidence to support firm recommendations for CT assessment in this context. “I think that's going to be a field where we will see a lot of research and where I would expect we would see the most significant change in perhaps an updated version [of the guidelines] in 5 or 6 years,” he said.
Additionally, “we may see automated techniques within postprocessing software based on integration of machine learning or artificial intelligence into the segmentation process, but that won't change the basic concepts,” Blanke said. “It won't change what kind of measurement or variable we are going to use to determine what valve to implant [in] the patient.”
Photo Credit: Philipp Blanke. Volume-rendered image of a balloon-expandable transcatheter heart valve in aortic position.
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Blanke P, Weir-McCall JR, Achenbach S, et al. Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI) / transcatheter aortic valve replacement (TAVR): an expert consensus document of the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr. 2019;Epub ahead of print.
Disclosures
- Blanke reports serving as a consultant for Edwards Lifesciences and Circle Cardiovascular Imaging, and providing CT core lab services for Edwards Lifesciences, Medtronic, Neovasc, and Tendyne Holdings, for which he receives no direct compensation.


Comments