No Stents in STEMI? With Careful Selection, Maybe
Data from DANAMI3-DEFER hint that it can be safe in some patients who have adequate flow and minimal residual stenosis.

It may be safe to omit stenting in some STEMI patients who have adequate flow restored and minimal residual stenosis, according to a look back at data from the DANAMI3-DEFER trial.
Yet researchers caution that their findings are hypothesis-generating only, since they stem from a post hoc analysis of a negative trial. DANAMI3-DEFER, which randomized more than 1,200 STEMI patients, found no benefit to PCI with deferred stenting over conventional PCI with immediate stent implantation. The new analysis, published online in EuroIntervention, focuses on patients in the deferred group who, due to their lesion characteristics, did not actually receive a stent and compares their outcomes with those of the immediate-stenting group.
Lead author Jasmine Melissa Madsen, MB (Rigshospitalet, Copenhagen University Hospital, Denmark), told TCTMD that the impetus for their analysis was not to decide which treatment strategy was better but rather to ask if, in some select, lower-risk cases, it’s safe not to implant a stent.
For lipid-rich plaques that rupture yet don’t cause significant stenosis, “should we actually be stenting this? It’s kind of equivalent to having an open wound—does it make sense to put a metal stent in a wound? No, not really,” she said. “[So] why do we do it anyway? We do it to prevent restenosis and secure the blood flow, obviously, but is that necessary with the good antiplatelet therapies that we have nowadays?”
Madsen pointed out that the answer to this question is especially relevant to younger people, such as those in their 40s, who have a STEMI. “When you implant a stent, you’re taking the risk of stent thrombosis and stent restenosis for the rest of that person’s life,” she observed.
Today, medicine is moving toward personalized treatment, so the findings from their analysis don’t apply across the board, she emphasized. “Obviously we still should be stenting [many] patients, but this study questions whether we should be stenting all patients. Because if there’s no obvious, underlying residual stenosis, why should we stent them?”
Ziad Ali, MD, DPhil (St. Francis Hospital, Roslyn, NY), agreed that the results are thought-provoking. “It’s a mounting level of evidence that I think supports the need for a more-controlled trial,” he commented to TCTMD. There’s growing data showing that the question of how best to intervene in STEMI “is more complicated and more nuanced than we thought.”
While there are some caveats to this analysis, “nonetheless it does reiterate the fact that perhaps we don’t need to stent lesions that are not stenotic and ischemia-producing, even in STEMI,” he commented to TCTMD.
Giulio Guagliumi, MD (Ospedale Papa Giovanni XXIII, Bergamo, Italy), also commenting on the study for TCTMD, described it as “interesting” but drew attention to its “highly selected population.” He cited the group’s overall short lesions, good LV function, and high prevalence of TIMI 3 flow at presentation. Additionally, he said, “I think that the criteria used for judging who is going to be suitable are, in my view at least, quite approximate.”
If stents are to be skipped, intracoronary imaging—which wasn’t done in DANAMI3-DEFER—could provide crucial information about which lesions are the best candidates, Guagliumi stressed.
A Special Subset
In DANAMI3-DEFER, 84 of 603 patients (13.9%) were randomized to deferred stenting but did not receive a stent. Of the 612 patients randomized to conventional PCI, 590 (96.4%) underwent immediate stenting.
Madsen et al stress that patients who didn’t get a stent were selected at the discretion of the operator: the group consisted of patients who had ≤ 30% residual stenosis evaluated visually by angiography, no significant thrombus, and no visible dissection. “Also, patients in whom it was necessary to stent immediately in order to restore a stable coronary blood flow are excluded from this cohort, and thus, the no-stenting approach is therefore selected for lower-risk clinical settings,” the researchers note.
Median stenosis in patients treated without stenting was 40%, with median vessel diameter of 2.9 mm and median lesion length of 11.4 mm. These individuals also were less likely than the immediate-stenting patients to have diabetes mellitus and hyperlipidemia, and they were less likely to receive prasugrel or ticagrelor at discharge.
Most of the unstented patients (71%) underwent plain old balloon angioplasty (POBA), while 7% had thrombectomy only and 21% underwent no intervention.
Over a median follow-up period of 3.4 years, the primary endpoint—a composite of all-cause mortality, recurrent MI, and TVR—was equally apt to occur in the no- and immediate-stenting groups (14% vs 16%; P = 0.85). After adjustment, there still was no significant difference by stenting strategy (HR 0.53; 95% 0.22-1.24). Nor were there differences in the individual components of the primary endpoint.
Switch the Paradigm?
Overall, Madsen said, their findings support the idea that opting not to stent “may be safe in some patients if you pick the right patients.”
The analysis also raises the possibility that deferred stenting does have an advantage, despite the negative results of DANAMI3-DEFER, because it provides a window of opportunity to see which individuals are good candidates for the no-stenting strategy, Madsen pointed out.
Moreover, avoiding stents means less manipulation of the culprit lesion, she added. “If you manipulate the lesion too much, you may end up with a lot of [distal] embolization causing a bad flow down your coronary arterial network, and this is something that you may avoid with the no-stenting approach.”
Going forward, Madsen said, studies could explore whether promising results from BASKET-SMALL 2, which showed noninferiority for drug-coated balloons in vessels with diameters below 3 mm, might extend to larger vessels if patients were carefully selected.
For Guagliumi, though, it’s not clear why there needs to be a paradigm switch when current DES have such good results. But operators who do weigh the option of omitting a stent, he said, need to proceed very carefully and must use some sort of intravascular imaging to ensure they have a clear understanding of the lesion characteristics at hand.
In DANAMI3-DEFER, lesions had to have residual stenosis ≤ 30% by visual estimation to be in the no-stenting group, but in fact QCA indicated that the median degree of stenosis following the procedure was 39.5%. “It is always difficult to judge when you have some thrombus inside,” which there could have been, given that operators were encouraged to not be overly aggressive with mechanical manipulation, said Guagliumi. Without imaging, he stressed, it is difficult to ascertain the amount of thrombus or instability.
It may be possible that some STEMI patients might present with a suitable plaque where stenting could be avoided, he allowed, “but in these cases I want to be certain about the measurements [and] about what we are leaving.”
As far back as 2002, the CADILLAC trial established that stents were superior to balloon angioplasty in acute MI, Guagliumi pointed out, and the gap between stents and balloons has only widened over the last two decades. “Today, we are using much better stents: thinner struts, much more biocompatible.” And in “the vast majority of countries” the price of these stents is not high, he said. But there is a cost to glycoprotein IIb/IIIa inhibitors, which were used in 40.5% of the no-stent patients, and thrombus aspiration is not recommended by current guidelines.
All that said, it’s “very reassuring” to see that event rates are very low in properly selected patients, Guagliumi agreed.
Ali pointed out that arguments for and against stenting can go both ways. Prior research has shown that by around 1 year of follow-up, event rates suggest “there don’t seem to be a lot of disadvantages to having a stent, but at the same time there’s not a lot of advantage to having a stent.” As such, “there’s going to be groups of interventional cardiologists that say, ‘I put in a stent, the lesion is completely resolved, and there’s no detriment.’ Others would say, ‘Why should I put any metal in the artery if I don’t have to? And if I have less than a 30% residual stenosis, I can leave it alone.’”
It’s known that there’s a 2% to 3% attrition rate of TLF with metallic DES after the first year, said Ali, but “we don’t know if that’s the same in a 30% lesion” as it would be in a more severely blocked vessel. Additionally, PROSPECT II showed that lesions with high lipid and plaque burdens will progress over time, he specified. “So the advantages and disadvantages of either strategy on long-term outcomes remains to be determined.”
Limitations of the DANAMI3-DEFER analysis include its lack of randomization and small number of patients, he said. Also, when interpreting the findings, it’s important to remember anatomical differences between the two groups. Side branches cover less myocardium, so skipping stents there may be less consequential, Ali pointed out, which might in part explain why this strategy was more common for the side-branch lesions in this trial. “I think when you’re talking about leaving a residual stenosis with a STEMI in a proximal LAD or proximal RCA circumflex, that’s very, very different from leaving a culprit lesion in a side branch.”
Plaque Composition Unknown
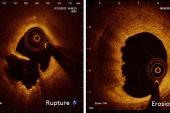
Prior studies like EROSION and EROSION III have suggested it’s possible to skip stenting in patients whose STEMI is caused by plaque erosion, not rupture. DANAMI3-DEFER unfortunately did not investigate plaque by optical coherence tomography (OCT), Madsen said. “It would have been ideal to have those data to clarify the exact etiology.” It’s possible that most of the no-stenting patients had STEMIs caused by eroded plaque, she said, though there’s no way of knowing that in hindsight.
In their paper, the researchers explore the mechanisms by which ruptured plaque, too, might be amenable to no stenting.
The lesions causing STEMI often start out with a luminal stenosis of less than 75% and may not limit coronary blood flow, they explain. “Thus, the risk of a plaque rupture with formation of thrombus and subsequent MI may not be determined by the degree of luminal stenosis, but by the plaque content.”
In particular, prophylactically stenting lipid-rich plaques that aren’t flow-limiting doesn’t seem to improve outcomes, they say, offering an explanation as to why: “Rupture of a vulnerable coronary plaque and exposure of underlying thrombogenic substrates in the lipid core, triggering thrombus formation, is known to be the underlying pathophysiology of STEMI. However, it is recognized that some of the ruptured plaques undergo spontaneous healing, modeling more-stable atherosclerotic lesions, and thereby remaining clinically silent and stable.”
This spontaneous healing subsequent to reopening of the coronary artery can occur without stenting, a process that could be further improved by dual antiplatelet therapy, the researchers say.
For Ali, one thing that’s novel about the DANAMI3-DEFER analysis is that it opens the door to the idea that both ruptured and eroded plaques could, if they meet the right criteria, be candidates for the no-stent approach. “The prevailing dogma has been that if a plaque ruptures to cap it with a stent is the safer thing to do, but there is really no evidence for that. If a plaque ruptures and the contents have embolized and the lumen is large and now the patient is put onto dual antiplatelet therapy, there is really no evidence to suggest that that plaque rupture wouldn’t heal in a safe way,” he said.
This could be good news for regions of the world where the cost of stents is prohibitive for some patients. “You have a small balloon, you can’t afford the stent, you have a less than 30% residual stenosis—you could say it’s safe for you to defer that patient according to this data,” Ali said.
Madsen, Ali, and Guagliumi all said that intracoronary imaging should be used to explore lesion characteristics and pinpoint which cases are best suited for no stents.
For Guagliumi, who has been at the forefront of OCT research, the case for skipping stenting is most compelling in an eroded plaque, though it can be hard to classify lesions even with imaging. “Artificial intelligence might help in detecting automatically which one is an eroded plaque in STEMI, and perhaps help to make a decision if you have enough lumen to avoid a stent,” he suggested. What’s needed now, he said, is a large randomized trial showing this could be done.
Caitlin E. Cox is Executive Editor of TCTMD and Associate Director, Editorial Content at the Cardiovascular Research Foundation. She produces the…
Read Full BioSources
Madsen JM, Kelbæk H, Nepper-Christensen L, et al. Clinical outcomes of no stenting in patients with ST-segment elevation myocardial infarction undergoing deferred primary percutaneous coronary intervention. EuroIntervention. 2022;Epub ahead of print.
Disclosures
- Madsen and Ali report no relevant conflicts of interest.



Comments