Nonstenotic Intracranial Plaque: An Underrecognized Source of Embolic Stroke
The optimal preventive strategies for this subset of patients with embolic stroke remain unclear, researchers say.
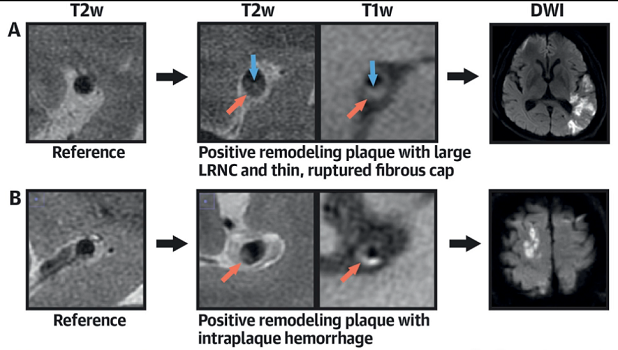
Photo Credit: J Am Coll Cardiol. Central Illustration (adapted).
High-risk, nonstenotic intracranial plaque appears to be a more important cause of embolic stroke of undetermined source (ESUS) than previously appreciated, according to findings from a Chinese imaging study.
Researchers led by Lin Tao, MD (General Hospital of Northern Theater Command, Shen Yang, China), showed that this type of plaque was more common and more vulnerable on the ipsilateral versus contralateral side, differences that were not seen in patients with acute ischemic strokes related to small-vessel disease (SVD).
“The present study provides the first evidence supporting an etiologic role for high-risk nonstenotic intracranial plaque in patients with ESUS,” they write, noting that “the optimal preventive strategies in these patients remain unclear.”
Senior author Hui-Sheng Chen, MD (General Hospital of Northern Theater Command), told TCTMD via email that future studies using high-resolution MRI techniques are warranted to “further define the specific subset of ESUS associated with intracranial high-risk plaque [and] inform the design of future clinical trials for stroke prevention.”
The study was published in the February 16, 2021, issue of the Journal of the American College of Cardiology.
Exploring the Causes of ESUS
ESUS, which represents up to one-third of ischemic strokes and carries a high risk of recurrence, has several potential causes. And in many cases, there can be more than one source.
Episodes of silent atrial fibrillation (AF) have been tagged as a potential major cause of ESUS, and two trials—RE-SPECT ESUS and NAVIGATE ESUS—explored whether use of direct oral anticoagulants (DOACs) instead of aspirin would reduce recurrent stroke risks. Both failed to show that, indicating that undetected AF might not be as important as a contributor to ESUS as originally thought.
Though prior studies have investigated extracranial plaque as a potential cause of ESUS, there have been none, aside from a case report, looking into the potential contribution of high-risk intracranial plaque, Tao et al say.
To explore the issue, they analyzed data on 243 patients with ESUS and 160 patients with ischemic strokes related to SVD who underwent 3.0-T high-resolution MRI to provide information on plaque morphology and composition.
ESUS patients were more likely to have any intracranial plaque compared with patients with SVD (68.7% vs 40.0%).
In patients with ESUS, the prevalence of intracranial plaque was higher on the ipsilateral versus contralateral side (63.8% vs 42.8%; OR 5.25; 95% CI 2.83-9.73). Greater plaque burden and remodeling index, as well as increased prevalence of positive remodeling, discontinuity of the plaque surface, and complicated plaque, also were seen on the ipsilateral side. In contrast, no such differences in plaque prevalence or vulnerability were observed in the SVD group.
Binary logistic analysis identified the remodeling index as the only factor independently associated with ESUS. With an optimal cutoff of 1.162, which corresponded to an area under the curve of 74%, the index had “good diagnostic efficiency” for ESUS, the researchers report.
“Positive remodeling, beyond luminal stenosis and plaque burden, reflecting an adaption of the artery behind the plaque, may play an important role in preserving lumen and harboring a large, vulnerable plaque in ESUS patients within intracranial lesions,” Chen said.
Implications for Treatment
In an accompanying editorial, Robert Hart, MD, and Kanjana Perera, MBBS (both Population Health Research Institute, Hamilton Health Sciences, Hamilton, Canada), say “this carefully performed, well-designed study using advanced imaging techniques and sophisticated statistical analysis establishes an etiological role for nonstenotic intracranial atherosclerosis in Chinese patients with ESUS.” They add that the findings are particularly relevant for patients in East Asian countries, where intracranial atherosclerosis is common.
The results of this study may help explain why RE-SPECT ESUS and NAVIGATE ESUS failed to establish a benefit of DOACs in the setting of ESUS, they say. “We believe that this was due to the relative infrequency of occult paroxysmal atrial fibrillation as the embolic source combined with an underestimated contribution of antiplatelet-responsive atherosclerotic plaque.”
Nevertheless, Hart and Perera argue, it’s too early to give up on ESUS as a therapeutic target. They point an exploratory analysis of the COMPASS trial suggesting that risk of cryptogenic stroke is reduced by the combination of low-dose rivaroxaban (Xarelto; Bayer/Janssen) and aspirin.
“Clinical trials testing the combination of low-intensity anticoagulation plus aspirin in ESUS patients appear warranted, in our view, by recent understanding of the spectrum of embolic sources in ESUS patients, the relative response of these sources to antithrombotic therapy, and the potential global impact to reduce the burden of stroke,” they say.
Perera noted to TCTMD that she is leading a pilot study, called CATIS-ICAD, to investigate the impact of adding rivaroxaban 2.5 mg twice daily to aspirin in patients who have had an ischemic stroke and have intracranial atherosclerotic disease.
Better treatments are needed for these patients, she said. She pointed to several possibilities beyond the addition of low-dose rivaroxaban, including more-intensive antiplatelet or statin therapy, as well as a greater focus on exercise and dietary modification.
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Tao L, Li X-Q, Hou X-W, et al. Intracranial atherosclerotic plaque as a potential cause of embolic stroke of undetermined source. J Am Coll Cardiol. 2021;77:680-691.
Hart RG, Perera KS. Intracranial atherosclerotic plaque and embolic stroke of undetermined source: another piece of the puzzle. J Am Coll Cardiol. 2021;77:692-694.
Disclosures
- The study was supported by grants from the National Key R&D Program of China and the Science and Technology Project Plan of Liao Ning Province.
- Tao and Chen report no relevant conflicts of interest.
- Hart and Perera report compensation for participation in the COMPASS and NAVIGATE ESUS randomized trials testing rivaroxaban, sponsored by Bayer AG.




Comments