Sodium Fluoride PET Bests CT for Predicting MI in CAD Patients
The post hoc results still need prospective validation but offer hints that PET could play a bigger role in MI prediction.
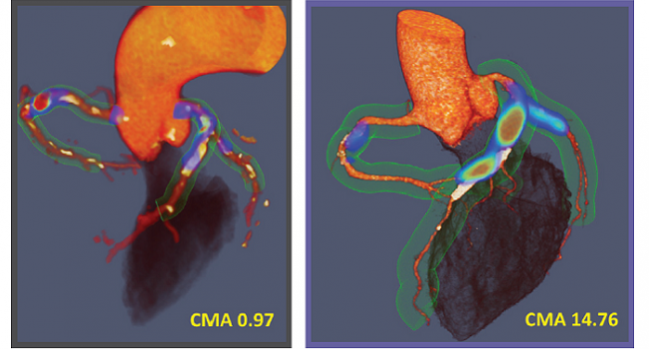
Positron emission tomography (PET) imaging using the calcification tracer 18F-sodium fluoride (NaF) appears to predict disease activity and MI risk in patients with known coronary atherosclerosis, according to a new post hoc study.
Current risk-stratification tools for secondary prevention are limited to risk scores, coronary calcium scoring, and severity indices of obstructive CAD; 18F-NaF has shown enough promise in bone imaging to warrant a closer look at its predictive capacity for MI and MACE, according to Piotr J. Slomka, PhD (Cedars-Sinai Medical Center, Los Angeles, CA), co-senior author of the paper. He told TCTMD that this tracer is already US Food and Drug Administration-approved and is “readily available” at most centers with CT imaging.
Pending prospective studies with this technology in the future, Slomka said he could imagine certain high-risk patients with established coronary disease and plaques seen on CT angiography undergoing additional 18F-NaF PET scanning in order to further risk stratify for secondary prevention. “And then [we could] perhaps decide about treatment,” he said. “There are some other CTA-based techniques and we'll have to probably confirm if sodium adds additional information compared to [them].”
The study, led by Jacek Kwiecinski, MD, PhD, and Evangelos Tzolos, MD (Cedars-Sinai Medical Center), was published in the June 23, 2020, issue of the Journal of the American College of Cardiology.
Better Risk Stratification
For the post hoc analysis, the investigators included 293 patients (84% men) from prospective observational studies. Most (79.1%) had stable disease while the rest were recruited and imaged following MI. The cumulative comorbidity burden was high with hypertension, hyperlipidemia, and tobacco use reported in 60%, 88%, and 67%, respectively. More than 90% were on statins, 90% were on antiplatelet therapy, and more than two-thirds were taking ACE inhibitors or ARBs.
On CT, the median calcium score was 334, with 20% having scores above 1,000. The median segment involvement score on CTA was five, with 74% having at least four segments involved.
PET scanning showed increased tracer activity in 70.9% of patients using the maximum target-to-background ratio (TBR) approach. Compared to those with no uptake, patients with increased 18F-NaF uptake had higher median values for the SMART clinical risk score (17 vs 19; P = 0.029) and coronary calcium score (184 vs 371; P = 0.0031), but no differences were observed in the presence or severity of obstructive coronary lesions. In total, 69.3% of patients showed increased 18F-NaF activity (coronary microcalcification activity [CMA] > 0) when looking at whole vessel microcalcification, with a median CMA value of 0.66.
Over 42 months, 20 MIs (primary endpoint) were reported—three fatal and 17 nonfatal—all of them among patients with increased coronary 18F-NaF activity. None of these patients had increased REACH or SMART clinical risk scores or coronary calcium scores, nor did they have an increased prevalence of obstructive CAD compared with those without MI.
On ROC analysis, fatal or nonfatal MI prediction was highest for 18F-NaF CMA, which outperformed coronary calcium scoring, modified Duke CAD index, and the REACH and SMART risk scores (P < 0.001 for all).
The specificity and sensitivity of 18F-NaF imaging among patients with CMA over 1.56 were 66% and 80%, respectively, for the primary endpoint. Moreover, on multivariate patients with CMA > 1.56 were at more than a sevenfold increased risk of MI independent of age, sex, risk factors, segment involvement and coronary calcium scores, presence of coronary stents, coronary stenosis, REACH and SMART scores, the Duke CAD index, and recent MI (adjusted HR 7.1; 95% CI 2.2-25.1; P = 0.003).
Patients with reported MACE also had higher CMA (median 1.9 vs 0.51; P = 0.0098) and a trend for higher TBR values (median 1.34 vs 1.22; P = 0.073) than those without.
“Calcium scoring has been shown in many publications to be a very powerful predictor of cardiac events, and yet in this population here—the patients with stable established coronary disease, so not people with suspected disease—calcium doesn't really predict events,” Slomka said. “It surprised me how well sodium fluoride separated considering we didn't have that many events here and this is a relatively small sample. For this kind of a prognostic study, we had a very clear separation from those CT-based measures, both the CT and CTA measures.”
He added that the reason for success observed here with 18F-NaF scanning is due to the fact that it can identify plaques at “very high risk of rupture” due to microcalcification. This was the prior working hypothesis and “we seem to confirm it in this prognostic study,” said Slomka.
Next Steps
The next step will be using this technology in larger studies with longer follow-up, Slomka said, adding that the ongoing 700-patient PREFFIR study should help bring some clarity. “Perhaps we'll be able to validate the thresholds established in this publication and show that this is applying these thresholds prospectively,” he said. “Then I think we need to start thinking about how to actually integrate sodium imaging into clinical decision-making and clinical protocols.”
Commenting on the paper for TCTMD, Ron Blankstein, MD (Brigham and Women's Hospital, Boston, MA), pointed out that how best to translate the findings to patient care is a key unanswered question.
“One of the challenges is that when you look at tracers like sodium fluoride or any other tracers, these are expensive scans to do. So before we start unrolling this and doing it in the general population to predict risk, we need to know not only that it predicts risk, but based on the results, [see] what kind of therapies would that lead us to and whether that ultimately changes outcomes,” Blankstein said. “So, there's a lot of questions there, and while I think it's a fascinating study, it's not one that paves the road for immediate clinical implementation at this stage.”
There's a lot of questions there, and while I think it's a fascinating study, it's not one that paves the road for immediate clinical implementation at this stage. Ron Blankstein
There is a clear need to further identify very high-risk patients who can benefit from secondary prevention. A current difficulty with 18F-NaF imaging is that while it has “really good sensitivity,” the specificity isn’t necessarily where it needs to be, Blankstein said. “That's a challenge because if we developed, let's say, a therapy based on this, at least if you're going to give it to anyone who has sodium fluoride uptake, that means treating 69% of the population, which is a pretty high number if you're talking about an expensive therapy that you want to limit only to the people who will have events.”
Additionally, this leads back to the ongoing debate in cardiology about whether “we should be identifying high-risk patients or high-risk plaques,” Blankstein noted. “We have shifted toward identifying high-risk patients because most of the therapies we use are systemic therapies that affect plaque throughout the coronary tree. . . . As a patient, if you have sodium fluoride in your coronaries, you're higher risk and maybe you need more aggressive therapies. But I think that the hope of potentially identifying high-risk plaque because it has sodium fluoride uptake and then going in and doing a revascularization and stenting that plaque is a concept that certainly will need a lot more research before we're able to adopt that.”
In an accompanying editorial, Zahi A. Fayad, PhD, and Philip M. Robson, PhD (both Icahn School of Medicine at Mount Sinai, New York, NY), write: “Having passed the milestone of demonstrating the ability of 18F-sodium fluoride PET imaging to predict clinical events, there is greater potential for this advanced noninvasive imaging to play an increasing role in the management of coronary artery disease, meeting the important unmet clinical need of risk stratification for patients with advanced coronary artery disease. The success of 18F-sodium fluoride, a cheap and readily available radiotracer, is significant in increasing the potential for more widespread use.”
The editorialists note that ongoing research on additional tracers like 18F-FDG and 68Ga-DOTATATE might also bear fruit in this realm.
Blankstein said he would like to see future studies “look at high-risk plaque characteristics and whether sodium fluoride adds on top of that. The reason why that's important is because coronary CT [angiography] is still going to be much more widely performed and far less expensive. So, I would want to make sure that this technique—which while very promising, is expensive—and I would want to make sure that it can really add on top of plaque characteristics by coronary CT angiography.”
Photo Credit: J Am Coll Cardiol. Central Illustration (adapted).
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Kwiecinski J, Tzolos E, Adamson PD, et al. Coronary 18F-sodium fluoride uptake predicts outcomes in patients with coronary artery disease. J Am Coll Caridol. 2020;75:3061-3074.
Fayad ZA, Robson PM. 18F-sodium fluoride PET imaging passes an important milestone toward noninvasive prediction of clinical events. J Am Coll Caridol. 2020;75:3075-3077.
Disclosures
- This research was supported in part by grants from the National Heart, Lung, and Blood Institute/National Institutes of Health.
- Slomka reports receiving software royalties from Cedars-Sinai Medical Center and receiving a grant from the National Institutes of Health.
- Kwiecinski, Fayad, and Robson report no relevant conflicts of interest.
- Blankstein reports receiving research support from Amgen and Astellas.


Comments