Tendyne Approved in US for High-risk Patients With Mitral Valve Disease
The approval is tightly defined: the system will be for patients ineligible for surgery and with anatomy unfavorable for TEER.
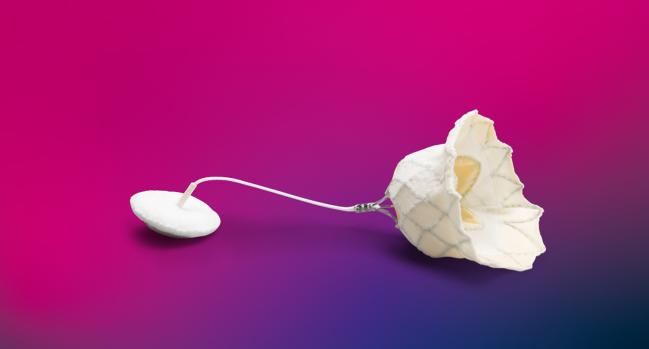
Photo Credit: Adapted from Abbott
The US Food and Drug Administration has approved the Tendyne transcatheter mitral valve replacement (TMVR) system for the treatment of patients with mitral valve dysfunction, device manufacturer Abbott announced today.
The approval is based on the SUMMIT trial, which included patients with severe mitral annular calcification (MAC) ineligible for cardiac surgery and those with symptomatic mitral regurgitation (MR) randomized to transcatheter edge-to-edge repair (TEER) with MitraClip or TMVR with Tendyne.
The indication is specifically for patients with moderate-to-severe MR, severe mitral stenosis, or moderate MR with moderate-or-greater mitral stenosis caused by MAC who are unsuitable for surgery and have anatomy deemed unfavorable for TEER.
In an Abbott press release, Paul Sorajja, MD (Minneapolis Heart Institute Foundation, MN), noted that MAC stiffens the mitral valve and can lead to MR or stenosis that disrupts the heart’s pumping ability. “Patients with MAC can be very difficult to operate on and many are considered too high risk for open-heart surgery due to multiple comorbidities or other factors,” said Sorajja.
According to European guidelines, the gold standard for primary MR is mitral valve repair surgery if the patient is not at high or prohibitive risk (class I, level of evidence B), but TEER can be considered in inoperable symptomatic patients with suitable anatomy (class IIb, level of evidence B). For secondary MR, TEER has a class IIa (level of evidence B) indication in selected symptomatic patients. The guidelines only mention that another transcatheter valve therapy might be considered in select cases when surgery or TEER isn’t an option (class IIb, level of evidence C).
In the US guidelines, TEER is considered reasonable in patients with primary MR and high/prohibitive surgical risk (class IIa, level of evidence B). It can also be considered in select patients with secondary MR (class IIa, level of evidence B). There are no current recommendations around TMVR.
In Europe, Tendyne was approved 5 years ago for use in patients with significant MR deemed ineligible for surgery or TEER on the basis of the Tendyne Global Feasibility Study.
Michael O’Riordan is the Managing Editor for TCTMD. He completed his undergraduate degrees at Queen’s University in Kingston, ON, and…
Read Full BioSources
Abbott. Abbott receives FDA approval for Tendyne, first-of-its-kind device to replace the mitral valve without open-heart surgery. Published on: May 27, 2025. Accessed on: May 28, 2025.





Comments