TRILUMINATE Pivotal Trial: First Data Support Safety, Efficacy of TriClip
In the trial’s roll-in cohort, 30-day major adverse events were few and 67% saw their TR severity dropped by at least two grades.
BOSTON, MA—Transcatheter edge-to-edge repair (TEER) using TriClip (Abbott) continues to show promise, with data from the TRILUMINATE Pivotal Trial’s roll-in cohort indicating the device has a low rate of major adverse events within 30 days in symptomatic patients with tricuspid regurgitation (TR).
The results, presented today during a late-breaking innovation session at TCT 2022, capture the learning curve associated with the device—participating centers could opt to include three patients each at the start of the randomized, controlled trial. In all, 97 patients were enrolled in this manner and by design received TriClip, out of 450 projected participants that will be randomized 1:1 to TEER versus medical therapy.
“The procedure is remarkably safe,” David H. Adams, MD (Mount Sinai Health System, New York, NY), told TCT attendees. Additionally, “the treatment was really quite effective, particularly when you consider the number of patients we treated with massive or torrential TR.”
Jörg Hausleiter, MD (Klinikum der Universität München, Germany), a TRILUMINATE investigator, explained that the pivotal trial’s protocol “allowed for a roll-in [for centers new to TriClip] to get a little more comfortable with the device and to really have at least some experience” ahead of their first randomized patient. A downside of this design is that data from these earliest cases will be included as part of the trial’s device arm. “I think we will see a learning curve for these rather complicated therapies,” Hausleiter predicted, adding, “It’s not only going in and placing the device,” but also involves preparing the patient and planning the procedure ahead of time.
He noted that two other ongoing studies of various transcatheter tricuspid valve treatments don’t feature the roll-in component: TRI-FR in France and TRICI-HF in Germany. “So it’s going to be very interesting to see if the variable experience going into such a trial might have an impact on the outcomes,” said Hausleiter.
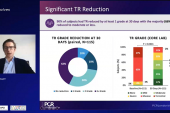
TR Reduced, While QoL and Function Improve
Of these 97 patients (mean age 79 years; 62% female), 91% had functional TR. Baseline TR was moderate for 2%, severe for 37%, massive for 24%, and torrential for 37%. Two-thirds were in NYHA class III/IV. Common comorbidities included atrial fibrillation (90%) and hypertension (80%), while 17% had previously undergone valve surgery.
Implant success reached 99%, and the mean device time (from steerable guide catheter placement in the right atrium until the retraction of the delivery system) was 105 minutes. An average of 2.3 clips were used per patient, with the majority (65%) receiving two clips. Most were placed anteroseptally.
Within 30 days, one patient had new-onset renal failure and died of cardiovascular causes (1%). There were no strokes/TIAs; one patient underwent tricuspid valve surgery and another required reintervention. Major bleeding occurred in seven patients (7.2%), including five who had GI bleeding.
By 30 days, 76% of patients were in NYHA class I/II, up from 32% at baseline. Kansas City Cardiomyopathy Questionnaire overall summary scores rose, on average, by 16.64 points.
In a core-lab analysis of 85 patients, 58% had massive or torrential TR at baseline, while 74% had moderate or less TR at 30 days. Nine in 10 saw their TR reduce by at least one grade, and two-thirds had a reduction of two or more grades. Notably, said Adams, “subjects beginning at massive or torrential TR were harder to get to moderate or less when compared to subjects with baseline severe TR.”
Going forward, severity of TR might be used to help identify which patients are the best candidates for TEER, he added. “Defining the expectations” will be important, Adams said. “I have no problem with a one-grade reduction of TR if that’s what the expectation is beforehand,” though more study is needed to know how much of a decrease in TR is sufficient to improve quality of life.
TriClip was granted CE Mark approval in Europe in 2020 based largely on the results of the single-arm TRILUMINATE study, and the newer G4 was cleared in 2021. Data from the bRIGHT registry also suggest the two iterations appear to be safe and effective in real-world use.
Caitlin E. Cox is Executive Editor of TCTMD and Associate Director, Editorial Content at the Cardiovascular Research Foundation. She produces the…
Read Full BioSources
Adams D. First report of outcomes in the TRILUMINATE Pivotal Clinical Trial of TriClip TEER in patients with tricuspid regurgitation: insights from the roll-in cohort. Presented at: TCT 2022. September 17, 2022. Boston, MA.
Disclosures
- Adams reports receiving royalties from and having intellectual property rights with Edwards Lifesciences and Medtronic, as well as receiving grant/research support from Medtronic, NeoChord, and Abbott Vascular to his institution.
- Hausleiter reports receiving fees related to consulting or speaking Edwards Lifesciences.




Comments