Patient ‘Progressing Well’ After Second Pig-to-Human Heart Transplant
The recipient, a 58-year-old man with end-stage heart failure, was not eligible for allotransplant.
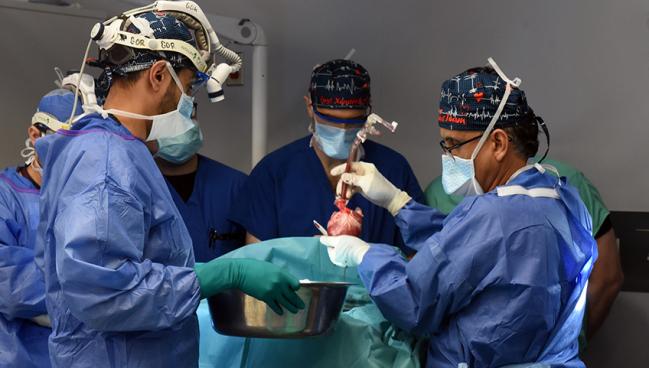
Photo Credit: Tom Jemski (University of Maryland School of Medicine Public Affairs)
A second patient with end-stage heart failure has undergone what surgeons are calling a successful transplant of a genetically modified pig heart and is now recovering well.
Last week's procedure mirrored an earlier surgery in January 2022, when the same team at the University of Maryland School of Medicine performed a xenotransplant in 57-year-old David Bennett. This time, the patient is receiving a novel experimental antibody called tegoprubart (Eledon Pharmaceuticals), a CD154 blocker, along with conventional antirejection drugs.
Lawrence Faucette, the 58-year-old recipient of the heart, was deemed ineligible for allotransplant due to preexisting peripheral vascular disease and internal bleeding.
“We are once again offering a dying patient a shot at a longer life, and we are incredibly grateful to Mr. Faucette for his bravery and willingness to help advance our knowledge of this field,” Bartley P. Griffith, MD (University of Maryland Medical Center, Baltimore), the lead surgeon in the most recent case as well as Bennett’s, said in a press release. “We are hopeful that he will get home soon to enjoy more time with his wife and the rest of his loving family.”
As of this point, “we are very confident that [Faucette] is making good progress,” Muhammad M. Mohiuddin, MD (University of Maryland School of Medicine), who co-led both operations with Griffith, told TCTMD. “Every day we take as a victory; we don't want to predict anything. And our main goal is to make this process beneficial to him, mostly, and for him to live a life that that he can enjoy.”
Every day we take as a victory. Muhammad M. Mohiuddin
In a press release, Mohiuddin said: “We are continuing to pursue the pathway to clinical trials by providing important new data on preclinical research that has been requested by the FDA. The FDA used our data from these new studies, as well as our experience with the first patient, to determine that we were ready to attempt a second transplant in an end-stage heart disease patient who had no other treatment options.”
Finding a Candidate
Mohiuddin said the team spent the better part of the last 6 months looking for an ideal candidate in whom to perform this procedure. Following the publicity around Bennett’s case, several patients had self-referred to the University of Maryland, but none of them quite fit the criteria they had in mind. Building off what they learned last year, the team wanted someone who was mobile, immunocompetent, and had not been hospitalized for a long period of time.
“It is very difficult to get a patient who is immunocompetent or has minimal other diseases,” he said. “Every patient has other diseases that make them ineligible for allotransplant. For us also, we don't want to take a patient who is terminally ill in a state that [when] we try them on immunosuppression, that will cause any harmful effect.”
Faucette was referred by a local cardiologist after being turned down for allotransplant or mechanical device implantation by multiple hospitals. The University of Maryland group submitted his application to the US Food and Drug Administration on August 29, 2023, and his xenotransplant was granted emergency approval on September 15, 2023, through its compassionate use pathway.
Lessons From the Past
After Bennett died 2 months following his xenotransplant, the team at University of Maryland School of Medicine concluded that a multitude of factors likely led to his death from heart failure, as outlined in an article published in the Lancet in June. They did not rule out that the presence of porcine cytomegalovirus in the pig heart may have contributed to the dysfunction of the transplant.
Based on this experience, Mohiuddin explained that several changes were made this time around. First, the team worked with Revivicor, the manufacturer of the genetically modified pig hearts, to develop better screening to identify the presence of porcine viruses. Also, the FDA mandated a rigorous surveillance protocol not only for the patient and his close contacts, but also for every healthcare worker who treats Faucette during his hospitalization. “So far, there is no evidence of any pig disease infecting humans, so we are very confident that this thing will not happen, and if it does, we think that our infectious disease group may be able to effectively manage it,” he said.
The new immunosuppression regimen, which has shown promise in phase II clinical trials for kidney transplant and amyotrophic lateral sclerosis, should also be more robust in helping prevent infection, Mohiuddin said. The old protocol targeted the same co-stimulation pathway between T and B cells by blocking the antibody itself, but this time they are going after the antibody ligand, he explained.
“We will be monitoring [these drugs] very closely—their levels will be monitored and the dosage and their frequency will be adjusted based on that,” he said, adding that they also learned from past experience not to stop immunosuppressants or antivirals or switch to a lower dose. “It's a learning experience for this drug in this procedure.”
Over the last 2 years, the field of xenotransplantation has continued to develop. Last July, a team from NYU Langone Health in New York, NY, announced it had successfully performed two cardiac xenotransplantation surgeries in brain-dead patients. Over the summer, another team from NYU successfully transplanted a genetically modified pig kidney into a brain-dead man.
The conversation about xenotransplant has entered into the forefront, with a 2022 review paper in JACC: Basic to Translational Science outlining the barriers that still need to be overcome. Namely, issues of rejection, infection, and physiologic differences between pig and human hearts are the most pressing. Social and ethical issues around the manufacture of these hearts, their cost, and deciding who should receive them abound as well.
The long-term goal for Mohiuddin and his team is a clinical trial, and they will continue to perform the animal studies required to get them there. “It may take more than a year or two,” he said. “However, in the meantime, a lot of patients like Mr. Faucette will lose their lives. So if we are able to do one-offs in the meantime and learn from it, that will benefit everyone.”
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
University of Maryland School of Medicine. UM Medicine faculty-scientists and clinicians perform second historic transplant of pig heart into patient with end-stage cardiovascular disease. Published on: September 22, 2023. Accessed on: September 26, 2023.




Comments