PROMISE II: Deep-Vein Arterialization Durable for Amputation-Free Survival in CLTI
Careful follow-up and good wound care are key to ensuring success in “no-option” patients, says Daniel Clair.

LAS VEGAS, NV—A device that diverts blood flow through arterialization of the deep veins in the foot shows continued durability in helping “no-option” patients with chronic limb-threatening ischemia (CLTI) avoid amputation, according to 1-year data from the PROMISE II study.
The rate of limb salvage at 1 year in the highly comorbid population, who were not candidates for surgery or endovascular revascularization, was 69%.
“The procedure is reproducible and generalizable,” said Daniel Clair, MD (Vanderbilt University Medical Center, Nashville, TN), in his presentation here at VIVA 2023. “Most operators in PROMISE II had essentially no experience in this technique, and I continue to believe that as we gain experience with this over time, we'll learn more and do better as we move forward.”
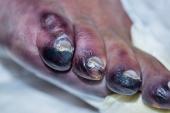
The average wound size following the procedure was 0.2 cm2. “So, you're talking about minimal wounds that these patients had, when they did have remaining wounds, much different than the 6.8 square centimeters that they started with,” he added.
Following the presentation, moderator Sean P. Lyden, MD (Cleveland Clinic, OH), noted that there was a significant reduction in pain scores from baseline to 1 year. This, he said, is particularly important in light of the fact that “many of the patients with no option generally eventually choose amputation because of pain.”
As Clair explained, the resolution of pain doesn’t happen overnight. Pain scores increased slightly in many patients after the procedure, but they declined within several months and continued going down.
To TCTMD, he said a small proportion of patients experience postprocedural swelling, which may account for some of the increased pain, as might venous hypertension.
“Normally when we do an arterial revascularization, their pain gets better very quickly. This doesn't happen with this procedure. The angiogenesis which occurs happens over 8 to 12 weeks, and during that time you need to have very careful follow-up for these patients,” Clair added.
I continue to believe that as we gain experience with this over time, we'll learn more and do better as we move forward. Daniel Clair
In September, the US Food and Drug Administration approved the LimFlow system on the basis of 6-month data from PROMISE II, making it the first and only FDA-approved device for transcatheter arterialization of deep veins. On November 1, 2023, LimFlow announced that it had entered into a definitive agreement to be acquired by Inari Medical.
The endovascular procedure involves creating an arteriovenous fistula proximal to atherosclerotic tibial arteries with the use of the LimFlow device, a self-expanding covered stent. This allows blood flow to be diverted, bypassing the area of atherosclerosis in the tibial artery and establishing arterial blood flow into the venous system of the foot, potentially avoiding above ankle amputation. As TCTMD has previously reported, amputation often leads to death within 2 to 5 years for the most-vulnerable CLTI patients, a disproportionate number of whom are Black and economically disadvantaged.
Poorer Outcomes in Dialysis Patients
For the single-arm PROMISE II study, Clair and colleagues screened 219 no-option CLTI patients from 20 centers in the United States. After evaluation, 105 individuals (median age 70 years; 31% female; 43% Black, Hispanic, or Latino) were deemed suitable for the procedure.
More than three-quarters of the cohort had diabetes, 91% had hypertension, 40% had chronic kidney disease (CKD), and 74% had undergone prior revascularization of the target limb. All had a nonhealing foot ulcer or gangrene and were Rutherford class 5 or 6. Patients were classified as “no-option” due to lack of runoff target for traditional intervention (97%) and lack of suitable conduit for bypass (3%). Arteriovenous crossing locations were posterior tibial artery in 76%, peroneal artery in 19%, and tibioperoneal trunk in 6%.
At 6 months, amputation-free survival (the primary endpoint) was 66% and the limb salvage rate was 76%, with approximately half of patients showing healing or healed wounds.
The 1-year data show a limb salvage rate of 69%, suggesting durability of the technique. In qualitative analysis, 92% of patients had wound healing and 45% had fully healed wounds at 1 year. Importantly, there was evidence of lower-extremity ischemia resolution, with more than 50% of patients in Rutherford class 0. On a pain scale of 0 to 10, the average pain score was 1.4.
One group who did not fare well overall with the technique was CKD patients on dialysis. Compared with patients not on dialysis, those who were had lower amputation-free survival (32% vs 61%; P = 0.0012) and a lower rate of overall survival (55% vs 86%; P = 0.0001).
Clair and colleagues also performed a pooled analysis to assess clinical outcomes of patients who underwent the LimFlow procedure in PROMISE II and well as the earlier PROMISE I trial. At 12 months, the survival in the pooled analysis was 86%, the limb salvage rate was 73%, and amputation-free survival was 63%.
Emphasis on Wound Care
Noting the lack of a comparison arm, panelist Eric Secemsky, MD (Beth Israel Deaconess Medical Center, Boston, MA), questioned whether there is a case to be made for a randomized trial to strengthen the evidence for the deep-vein arterialization technique.
“There is the potential for a randomized trial, but I do think it’s very difficult to randomize patients who don't have an option that we can offer them that [matches] what we're able to do now. It's going to be difficult in this environment,” Clair said.
Panelist Venita R. Chandra, MD (Stanford University Medical Center, CA), added that the poorer outcomes in the dialysis patients could be an argument against offering the treatment to them unless there is a proven pain benefit.
“When we think about alternatives, the alternative here is either management with medical therapy, which will involve continued pain in my estimation, or amputation,” Clair noted. “It's important to remember that amputation comes with a 5 to 10% perioperative mortality, which is very different from this.”
Expanding on his earlier comment about operators picking up the procedure easily even with no experience in it, Clair told TCTMD that the real question now is not whether people can do successful deep-vein arterialization, but whether they can successfully manage the follow-up wound care.
“Honestly, the concern with this technology is if everybody just starts doing this and doesn't modify the way they deal with patients afterward, I think we're not going to see the success rates that we're seeing in this trial,” he cautioned.
L.A. McKeown is a Senior Medical Journalist for TCTMD, the Section Editor of CV Team Forum, and Senior Medical…
Read Full BioSources
Clair D. 12-Month Results from the PROMISE II US pivotal trial of the LimFlow system. Presented at: VIVA 2023. October 31, 2023. Las Vegas, NV.
Disclosures
- The trial was sponsored by LimFlow.
- Clair reports honoraria from Boston Scientific and Endologix; and consulting for Boston Scientific, Caeli Vascular, ElastiMed, Endologix, LimFlow, Medtronic, Nectero, and Vesteck.



Comments