QCA an Alternative to IVUS? GUIDE-DES Trial Hints at the Possibility
Though they come with caveats, the results suggest QCA could fill a niche for operators who don’t use intravascular imaging.
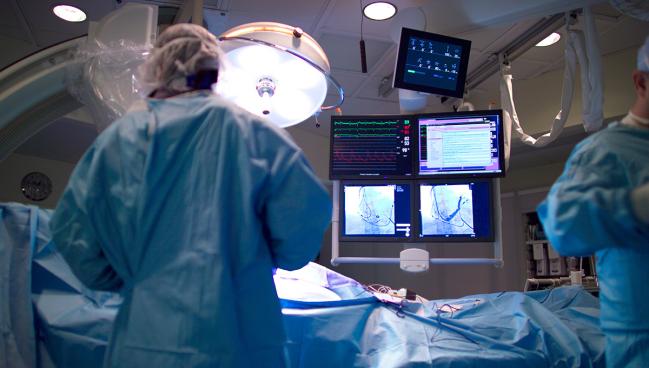
When used systematically, quantitative coronary angiography (QCA) may be a reasonable alternative to intravascular ultrasound for guidance during percutaneous coronary interventions, new data from the GUIDE-DES randomized trial suggest.
The two modalities produced similar rates of target lesion failure at 12 months, but given that events were less frequent than expected, the researchers urge caution in interpreting the results.
Time and again, randomized trials have shown intravascular imaging with IVUS or OCT can be helpful in guiding DES implantation, especially for complex cases, though evidence has been mixed as to whether there’s an impact on hard outcomes.
But these comparisons were against conventional angiography, not QCA, Cheol Whan Lee, MD (University of Ulsan College of Medicine, Seoul, Republic of Korea), pointed out to TCTMD.
“In these clinical trials, angiography-guided PCI relies on visual estimation, resulting in high inter- and intraobserver variabilities and often leading to suboptimal results,” such as small minimum stent area and edge problems, Lee noted in an email. “Furthermore, high-pressure postdilation after stenting to minimize residual stenosis [has been] performed less frequently in angiography-guided PCI.”
What their new data show is that these issues “are not insurmountable,” he said. “We believe that ‘QCA-guided PCI with routine . . . postdilation’ may overcome the drawbacks of conventional visually guided PCI and help improve PCI outcomes when IVUS is unavailable.”
The results were published online recently in JAMA Cardiology.
TLF Similar at 12 Months
Led by Pil Hyung Lee, MD (University of Ulsan College of Medicine), the GUIDE-DES investigators analyzed data on 1,528 patients randomized to undergo PCI with QCA guidance (mean age 64.1; 75.2% male) or IVUS guidance (mean age 64.6 years; 81.3% male). After PCI, the minimal lumen diameter was similar in the two groups (mean 2.57 and 2.60 mm, respectively).
Researchers had designed the trial with an assumed target lesion failure (cardiac death, target-vessel MI, or ischemia-driven TLR) rate of 8% at 12 months, where the upper limit of the one-sided 97.5% CI would show an absolute difference of less than 3.5 percentage points for noninferiority.
By 12-month follow-up, however, target lesion failure had occurred in just 3.81% of the QCA group and 3.80% of the IVUS group (P = 0.99), with no differences seen across various subgroups. The QCA and IVUS study arms also had statistically similar rates of stent edge dissection (1.2% vs 0.7%), coronary perforation (0.2% vs 0.4%), and stent thrombosis (0.53% vs 0.66%).
No More ‘Guesstimation’
Ajay J. Kirtane, MD (NewYork-Presbyterian/Columbia University Irving Medical Center, New York, NY), and Roxana Mehran, MD (Icahn School of Medicine at Mount Sinai, New York, NY), both associate editors at JAMA Cardiology, co-authored an Editor’s Note to accompany the paper.
They observe that despite the evidence for intravascular imaging, as well as support from consensus documents, the technology continues to be “used in less than 25% of PCI procedures worldwide due to clinical inertia, cost and reimbursement considerations, and other factors.”
Before catheter-based imaging’s debut in the field, though, first came efforts to standardize lesion assessment with angiographic methods like QCA, a technology that Kirtane and Mehran concur could fill a niche.
“Online use of QCA does require additional measurements and briefly interrupts catheterization laboratory workflow,” they acknowledge. “However, QCA adds no additional costs to PCI procedures and is a widely available and included component of catheterization laboratory equipment—yet, it is almost never used in clinical practice. Accepting that we need to move beyond visual ‘guesstimation’ to improve PCI outcomes, the ready availability and potential cost savings of an angiography-based strategy for PCI guidance could help to surmount the activation energy needed to incorporate routine catheter-based intravascular imaging within practice.”
Lee agreed that QCA could play a valuable role in procedural guidance. With intravascular imaging, knowledge of plaque characteristics and vascular dimensions can both aid in planning PCI. The latter is critical for stent selection and optimization, he explained.
“Although QCA is considered inferior to IVUS (OCT) for vascular sizing, it is certainly better than visual estimation. The imaging catheter for IVUS (OCT) should be parallel to the arterial wall to obtain a cross-sectional image; otherwise, it is not the true diameter. Considering that there is no precise value, only an approximate one, for the diameter measured by IVUS as well as OCT, I believe that QCA is reliable and acceptable for PCI guidance.
“However, further comparative studies between imaging- and QCA-guided PCI may be needed to persuade interventionists to use QCA guidance in daily PCI practice,” Lee emphasized.
As for interpreting the current study, Kirtane and Mehran have some caveats. In particular, the QCA strategy involved “a somewhat unconventionally aggressive sizing algorithm with mandatory routine postdilation of stents; this clearly distinguishes it from a more conventional PCI procedure,” they write. Moreover, the low event rates mean the noninferiority of QCA can’t be ascertained from the GUIDE-DES data, and there’s no direct comparison of either QCA or IVUS versus subjectively assessed angiography.
Lee, too, specified that operators participating in GUIDE-DES followed a strict protocol. “Systematic, step-by-step procedural methodological details for both modalities may be needed to ensure a fair comparison between the two different procedures,” he said.
The information that QCA provides can vary based on calibration points, angiographic views, and vessel size. “This variability might make interventionists hesitant to adopt it, emphasizing the need for practical and reliable approaches to overcome its limitations,” said Lee.
For operators who do use QCA, they recommend “measurement of reference vessel (landing zone) diameters and lesions using the usual practice view without vessel foreshortening and branch overlapping,” he said. “While a single QCA measurement at the usual practice view is generally acceptable, obtaining QCA measurements at two different views, including caudal views, may be preferable for the left main and the nearby section (within 10 mm from the ostium) of the left anterior descending coronary artery. Take a large diameter for PCI guidance if multiple QCA measurements are done.”
Looking to the future, Lee said a promising method is three-dimensional and artificial intelligence-assisted QCA, which enables full visualization of the coronary artery.
Caitlin E. Cox is Executive Editor of TCTMD and Associate Director, Editorial Content at the Cardiovascular Research Foundation. She produces the…
Read Full BioSources
Lee PH, Hong SJ, Kim H-S, et al. Quantitative coronary angiography vs intravascular ultrasonography to guide drug-eluting stent implantation: a randomized clinical trial. JAMA Cardiol. 2024;Epub ahead of print.
Kirtane AJ, Mehran R. Moving beyond conventional guesstimation to guide PCI. JAMA Cardiol. 2024;Epub ahead of print.
Disclosures
- Biotronik funded the investigator-initiated GUIDE-DES trial.
- Pil Hyung Lee reports receiving grants from Abbott Cardiovascular, Boston Scientific, Medtronic, and Dong-A ST outside the submitted work.
- Cheol Whan Lee reports receiving a grant from Biotronik during the conduct of the study.
- Kirtane reports institutional funding to Columbia University and/or the Cardiovascular Research Foundation from Medtronic, Boston Scientific, Abbott Vascular, Amgen, CathWorks, CSI, Philips, Recor Medical, Neurotronic, Biotronik, Chiesi, Bolt Medical, Magenta Medical, Canon, SoniVie, and Shockwave Medical and personal fees from Amgen, Medtronic, Biotronik, Boston Scientific, Abbott Vascular, CathWorks, Concept Medical, Edwards, CSI, Novartis, Philips, Abiomed, Recor Medical, Chiesi, Zoll, Shockwave, and Regeneron.
- Mehran reports receiving grants from Abbott, Abiomed, Affluent Medical, Alleviant Medical, Amgen, AM-Pharma, Arena, AstraZeneca, AtriCure Inc, Biosensors, Biotronik, Boston Scientific, Bristol Myers Squibb, CardiaWave, CeloNova, CERC, Chiesi, Concept Medical, Cytosorbents, Daiichi Sankyo, Duke, Element Science, Essential Medical, Faraday, Humacyte, Idorsia, Janssen, MedAlliance, Medscape, Mediasphere, Medtelligence, Medtronic, MJH Healthcare, Novartis, OrbusNeich, Penumbra, PhaseBio, Philips, Pi-Cardia, PLx Pharma, the Population Health Research Institute, Protembis, Recor Medical Inc, RenalPro, RMGlobal, Sanofi, Shockwave, Vivasure, and Zoll; receiving personal fees from Affluent Medical, the Cardiovascular Research Foundation, Cordis, Daiichi Sankyo Brasil, E.R. Squibb & Sons, Esperion Science/Innovative Biopharma, Europa Group/Boston Scientific, Gaffney Events, Educational Trust, Ionis Pharmaceuticals, MedCon International, Novartis, Novo Nordisk, PeerView Institute for Medical Education, Terumo Europe NV, Vectura, VoxMedia, IQVIA, Radcliffe, TARSUS Cardiology, and WebMD; having equity in Applied Therapeutics, Elixir Medical, Stel, and ControlRad (spouse); receiving nonfinancial support from the American Medical Association (scientific advisory board member) and the Society for Cardiovascular Angiography and Interventions (Women in Innovations committee member); serving on the faculty of the Cardiovascular Research Foundation; and receiving honoraria from JAMA Cardiology (Associate Editor) and the American College of Cardiology (board of trustees member, steering committee member of the Clinical Trials Research program).



Comments